
A look at some best practices to ensure that quality control is maintained in the client–vendor relationship.

A look at some best practices to ensure that quality control is maintained in the client–vendor relationship.

Only careful planning can prevent problems that stem from differences between a sponsor’s and a CDMO’s equipment, practices, and culture. This article highlights best practices and case studies.

SGS announces expansion of cell bank and bulk harvest testing services.

CDMO leader Marc Funk will succeed Ridinger as CEO of Lonza.

Fujifilm increases capacity of its Bio-CDMO business with an expansion of production in North Carolina.

The $252-million acquisition is expected to strengthen Cambrex’s position as a small-molecule CDMO across the drug lifecycle.
Requirements for virus filtration must be considered in developing continuous downstream processes.
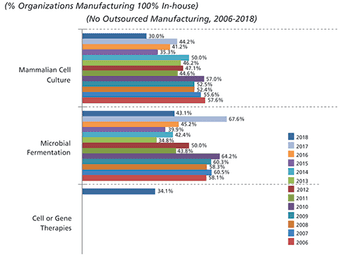
Outsourcing of manufacturing activities is expected to increase in 2019.

While pharma is proving its capabilities to develop novel therapies, the industry still needs to work on manufacturing innovation.

Keeping valuable employees happy-and on the job-may test bio/pharma business decisions.

Intellectual challenge, work/life balance, compensation, and an unclear business outlook create uncertainty among European bio/pharma employees.

The company is investing approximately $14 million to expand biologics packaging capabilities and capacity at its biologics manufacturing facility in Bloomington, IN.

CMOs and CDMOs made investments in new and expanded facilities and services in the last quarter of 2018.

The contract research organization has increased its US-based early phase clinical capacity and doubled its specialty lab space.

Catalent will expand primary packaging capabilities and commission an automated, high-speed bottling line for clinical packaging of capsules and tablets.

ADC Biotechnology will invest downstream formulation, fill/finish capabilities, and Lock-Release conjugation technology.

With the $252-million acquisition of contract development, manufacturing, and testing organization Avista Pharma Solutions, Cambrex will enter the market for early stage small-molecule development and testing services.

As biopharma companies rapidly change their focus, they may lack the laboratory space, instrumentation, and the scientific knowledge to support biologics research.

Ardena has moved into its expanded headquarters, located in Gent, Belgium, as a result of continued growth.

The expansion adds new capabilities and enhances existing service offerings for both oral and parenteral dosage forms.

The growth in adoption of single-use systems for commercial manufacturing will be dramatic in coming years.

Survey results and record attendance show positive signs for various bio/pharma regions.

The company will complete an expansion of its secondary packaging capabilities at its Ravensburg, Germany site by 2020.

Cambrex will create a new center of excellence for API process development and clinical supply at its High Point, NC facility and expand its API manufacturing facility in Italy.

Clovis Oncology and Lonza celebrated the grand opening of a dedicated production train at Lonza’s Visp, Switzerland site for manufacturing Rubraca (rucaparib).