
Innomech has developed an innovative new test station for Fluidic Analytics to streamline R&D programs. The new test station serves as a labor-saving R&D tool to help process multiple test samples and to ensure product quality.

Innomech has developed an innovative new test station for Fluidic Analytics to streamline R&D programs. The new test station serves as a labor-saving R&D tool to help process multiple test samples and to ensure product quality.

A $5.5-million expansion at its Philadelphia, PA clinical supplies facility gives Catalent additional packaging and storage capacity.

FDA sent a warning letter to Tris Pharma Inc. after investigators found the company had failed to properly investigate batch failures and establish quality control procedures.
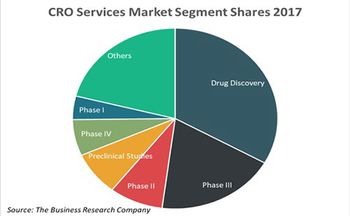
A new study by the Business Research Company reveals prominent contract research organization outsourcing trends.

The new 300,000-square-foot facility is considered the largest dedicated cell and gene therapy manufacturing facility with fully integrated services.

Anthony Qu, PhD, vice president of Scientific Affairs at Halo Pharma, will give a presentation on fixed-dose combination products, drug products containing multiple active ingredients, as an effective approach for simplified dosing at CPhI North America on Wednesday, April 25, 2018 in Philadelphia, PA.

The contract manufacturing organization’s facility in Boulder, CO, has passed general inspection from FDA.

On Tuesday, April 24, 2018, Evan Boswell, senior principal scientist at Pfizer CentreOne Contract Manufacturing, Pfizer CentreOne will give a presentation on scaling up the manufacturing process of active pharmaceutical ingredients at CPhI North America in Philadelphia, PA.

The contract development and manufacturing organization announced the addition of a new building complex that will house its headquarters in Bothell, WA.

Fujifilm acquires cell culture media companies Irvine Scientific Sales Company and IS Japan.

The active pharmaceutical ingredient and excipient provider has expanded its parenteral ingredient manufacturing capacity and lab services.

The contract service provider will invest in a laboratory expansion at its site located in Tredegar, Wales, UK.
Particle engineering using jet milling or spray drying can be used to obtain appropriate particle characteristics for inhalation drug products.

Access to multiple analytical techniques is essential for fully characterizing complex protein formulations.

CMOs/CDMOs that are easy to work with, have demonstrated performance track records, and plan for the future are preferred.

Industry analyst Jim Miller will provide insight into the past, present, and future of the contract services market at the CPhI North America conference on Wednesday, April 25, 2018 in Philadelphia, PA.

Certificates of analysis can be used to monitor the reliability of products and their suppliers, says Susan Schniepp, distinguished fellow at Regulatory Compliance Associates.
Formulation expertise can smooth the transition of a prospective therapy from medicinal chemistry to drug dosage form.

The contract research organization has added a new analytical laboratory in Middleton, WI, to expand its biologics testing capacity.

MedPharm has received a multi-million dollar investment by Ampersand Capital Partners to diversify its service offering and regional coverage.

Hitachi will manufacture regenerative medicines developed by Daiichi Sankyo and SanBio Group.

The agreement gives IncoCell Tianjin, a wholly-owned subsidiary of China-based Boyalife Group, access to Cesca’s celluar processing contract development and manufacturing services.

The company plans to install 4000-L disposable bioreactors from ABEC at its new commercial manufacturing facility in Wuxi city, China.

An expanded pharma services supply chain facility in Germany boosts Thermo Fisher’s Pharma Services footprint in Europe.

Hovione plans to continue to expand its sites in Portugal and Ireland for chemical synthesis, spray drying, and laboratory analysis.