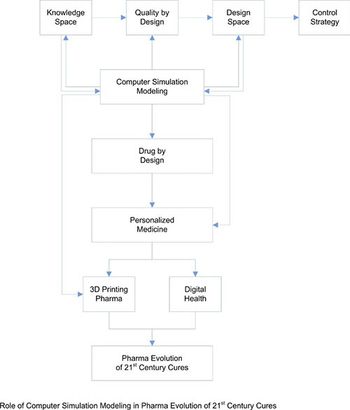
Twenty-first century drug development challenges sponsors and contract partners to collaborate more closely, using tools such as model-based simulation.

Twenty-first century drug development challenges sponsors and contract partners to collaborate more closely, using tools such as model-based simulation.

Catalent completes $4.6 million expansion at Singapore clinical supply facility and marks 20 years in the region.
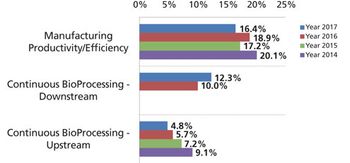
Development and adoption of new technologies create challenges that may take years to resolve.

Assessing potential formulation and manufacturing issues in early development phases can improve a drug’s chances for success.

Robert Iser of PAREXEL Consulting answers questions regarding the regulatory expectations of quality agreements and how companies can ensure the quality and safety of their products.

The contract development and manufacturing organization has entered it first manufacturing contract worth $148 million for its recently completed Plant 3 facility.

Avid Bioservices will provide commercial manufacture of an enzyme replacement therapy by Roivant Sciences' Enzyvant subsidiary.

The company aims to add the additional analytical services in the European Union throughout 2018.

Automation systems, vehicles, and robots improve efficiency of transporting materials and finished goods in pharmaceutical warehouses.

A new suite for the encapsulation of highly potent drugs will be added.

Unlike current approaches where bioconjugation is typically done following the manufacture of the monoclonal antibody and the cytotoxic drug, the new method begins with the antibody supernatants and eliminates the need for extensive chromatographic purification.

A $800-million acquisition of MPI Research expands Charles River's offerings for early-stage contract research.

The companies will partner to further immuno-oncology research using humanized mice.

Layout and supply details must be considered when implementing a fully disposable biopharmaceutical manufacturing process.

Hospitals form not-for-profit drug company to combat drug shortages and high prices.

Mark Egerton, PhD, chief executive officer of Quotient Sciences, shares insights on a new approach to accelerate drug development, which integrates formulation development, real-time adaptive GMP manufacturing, and clinical research within a single platform.

Industry lessons from a fast-track technology transfer of a soft-gelatin capsule (softgel) are multifold. This case study reviews the success factors for effective execution of the technology transfer, which include: strong relationship between the customer, the contract development and manufacturing organization, and other partners based on deep knowledge in the technology; established and proven quality-by-design processes; risk mitigation management; project leadership; flawless execution; and mutual trust.

Cambrex invests in development and laboratory facilities and adds staff.

Avista Pharma's strengthens early phase drug development offerings with acquisition of Solid Form Solutions.

Biopharm industry veteran Ralf Otto named to lead development and manufacturing at Rentschler Biopharma.

Chemical engineers and chemists each bring unique skills to API process development and optimization. Success depends on their close collaboration, at both sponsor and CDMO facilities.
A different perspective on controlling fixed costs of biomanufacturing, based on know-how from other industries, provides a competitive edge, says the CEO of Samsung BioLogics.

Successful outsourcing relationships in early phase drug-development analytics are driven by partnership.

CRLs put the brakes on drug development and damage corporate reputation and stock prices. Upfront investment and better sponsor oversight are ways to prevent them.
This article describes how to improve the regulatory journey from classification to market clearance for sponsors of medical device combination products.