
Also: DSM's North Carolina facility receives SafeBridge certification; Dynavax CFO to retire; more...

Also: DSM's North Carolina facility receives SafeBridge certification; Dynavax CFO to retire; more...

The US Food and Drug Administration released its Guidance for Industry titled Pharmaceutical Components at Risk for Melamine Contamination.

Soelkner discusses the latest industry developments and trends.

Also, Pfizer forms two research agreements in China; NanoInk appoints John Kubricky to its scientific advisory board; more...

As the pharmaceutical industry looks to emerging markets, corruption becomes an important issue.

Brief pharmaceutical news items for August 2009.

Biotech firms must first close the gaps between science and biology on the path toward QbD.

Individuals and companies at the top seem to have no problem short-circuiting their success.

Determined to prevent further supply-chain breaches, industry takes charge, offers proposals.

As the pharmaceutical supply chain expands, sponsor companies need to weigh all their options.
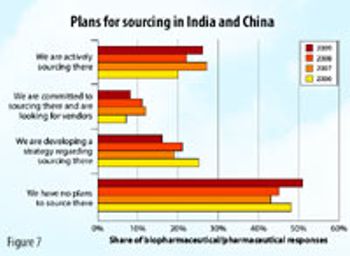
The contract services industry may not be as robust in 2009 as it has been in previous years, but it's not as bad as many people think.

A roundtable with pharma majors Pfizer and Johnson & Johnson, moderated by Jim Miller.
The author provides advice about evaluating contract analytical laboratories and establishing an effective procedure for working with them to perform reliable stability studies.

A Q&A with Pfizer CentreSource, moderated by Jim Miller.

Leading contract manufacturing organizations share their views on the current and future market dynamics shaping pharmaceutical outsourcing.

Also, Isogen completes sterile facility; Dishman appoints head of generic API business; FDA releases final rule on authorized generic drugs and NDA submissions; more...

China's State Food and Drug Administration has completed a new draft of GMP guidelines, but after a series of quality-control events, it will take time for the country to regain the global pharmaceutical industry's trust.

Also, Gilead and Tibotec form agreement; FDA approves 2009-2010 flu vaccine; Catalent appoints VP in packaging unit; more...

Also, Lonza and Medarex sign agreement; Covance appoints VP and chief scientific officer of global analytical services; more...

Also, Lundbeck acquired LifeHealth; FDA is seeking a director of its new tobacco regulation branch; Charles River Labs announces personnel changes; more...

A joint biopharmaceutical manufacturing facility in India by Kenwell and Boehringer Ingelheim ushers in new era.

Also, Wyeth and Catalyst sign agreement; FDA seeks public opinion about tobacco regulation; Catalent appoints VP of quality and regulatory affairs; more...

This week, the US Pharmacopeial Convention and the Vietnamese Pharmacopoeia Commission signed a memorandum of understanding that will help ensure the safety of Vietnamese medicines.

The regulators are doing it. But industry's fear of sharing information may leave them behind.

Is it good policy to pay for bad behavior?