
This article is part of a Special Report on the Emerging Markets of The East, October 2009

This article is part of a Special Report on the Emerging Markets of The East, October 2009

Company and People Notes: Boehringer Ingelheim will acquire Wyeth's animal health business; Amsterdam Molecular Therapeutics appoints CEO; more...

Julian Mosquera, Director of LCP Consulting, advises how pharma companies can make their supply chains leaner.

Despite the economic downturn, Spain's pharmaceutical industry continues to grow - particularly when it comes to biotechnology.

Company and People Notes: Wyeth and Ambrx form development pact; Elite Pharma appoints CEO and CSO; more...

Company and People Notes: Sanofi Pasteur signs H1N1 vaccine deal with Brazilian government; Helsinn Group appoints CEO of US business; more...

Small-format packaging equipment can provide benefits to the pharmaceutical industry such as quick changeover and low tooling costs. The machines also can shorten the time it takes to bring a product to the market.

Company and People Notes: Neoprobe and Laureate Pharma form manufacturing agreement; Akela Pharma appoints CEO and chairman; more...

The US Pharmacopeial Convention (USP) and the Permanent Commission of the Pharmacopeia of the United Mexican States (FEUM) signed a memorandum of understanding (MOU) last week.

Also, Orexo and Novartis form agreement; Affitech appoints Robert Burns CEO; more...

Brief pharmaceutical news items for September 2009.

After years of promomting QbD concepts, FDA's ready to take action on nonconformers.

Contract-service providers are expanding their offerings in this slow-growth environment.
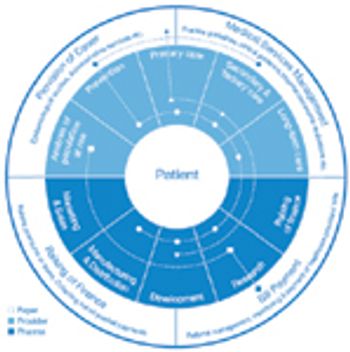
Personalized medicine and integrated healthcare delivery require new business and pricing models. This article contains bonus online-exclusive material.

Select contract manufacturing organizations roll out expansions for production of active pharmaceutical ingredients and intermediates.

As new process validation guidelines emerge, industry needs to reinvent how it releases product.

IPEC's new stability testing guide takes into account the full supply chain's storage conditions.
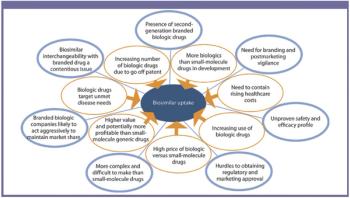
The author reviews the major biopharmaceutical markets' activity and predicts how the markets may evolve.
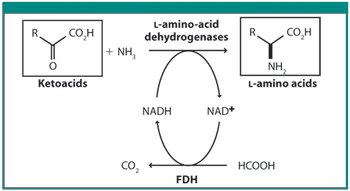
Scientists from DSM and Kaneka discuss various techniques in this roundtable moderated by Patricia Van Arnum.

Quanticate's Commercial Director explains the ins and outs of outsourcing.

In the current slow growth environment, service providers are looking to tactical expansions, as well as more ambitious strategic service expansions.

Increase your chances of success in the biggest market in the world.

Belgium is one of the largest centres for pharmaceutical distribution and has the second highest number of pharma exports per capita worldwide.

Eastern Europe's pharmaceutical market is forecast to grow at a CAGR of more than 10% to be worth more than $41 billion by 2014, according to a report from companiesandmarkets.com.

Company and People Notes: UCB and Novartis form agreement; AAIPharma appoints VP of regulatory affairs; more...