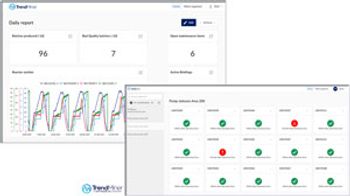
TrendMiner 2019.R3 displays a customized, actionable dashboard with a real-time view of the production process.

TrendMiner 2019.R3 displays a customized, actionable dashboard with a real-time view of the production process.

Nephron Pharmaceuticals is partnering with Clemson University to create a robotic solution for syringe-filling automation to enhance sterile manufacturing.

NIIMBL announced it is partnering with FDA through the University of Delaware to improve biopharmaceutical manufacturing.

Lonza plans to implement MES software to accelerate paperless, 24/7 production of drug capsules.

Process Automation Solutions becomes the latest partner to join the TrendMiner ecosystem.

Rockwell Automation’s updated FactoryTalk AssetCentre software supports several new types of production assets.

To rapidly achieve high-quality pharmaceutical manufacturing processes, industry must develop prospective, management-based approaches instead of retrospective performance-based measures.

Automation promises to connect biomanufacturing processes more closely, and to bring greater efficiency to the manufacturing floor.

A presentation at the Leistritz Pharmaceutical-Nutraceutical Extrusion Seminar explained how simulation tools benefit the development process.

GE Healthcare launches Chronicle GMP-compliant automation software for cell therapy manufacturing.

The Allen-Bradley ControlLogix 5580 controller from Rockwell Automation is compliant with the IEC 62443-4-2 security standard.

The FactoryTalk Analytics LogixAI module from Rockwell Automation detects production anomalies.

A data exchange program between GE Healthcare and Amgen aims to improve biologics manufacturing through analysis of how raw materials affect the process.

The UK’s Centre for Process Innovation is collaborating with partners GSK and AstraZeneca to establish a continuous wet granulation manufacturing facility for small-scale development of oral solid-dosage pharmaceuticals.

The Quartic Platform is a new, AI-powered smart manufacturing platform that can be integrated into legacy facilities and manufacturing processes.

Moving from paper-based to digitalized processes is the first step to enabling quality management and manufacturing to work in sync.

The GE Healthcare and Rockwell Automation collaboration will help meet the needs of the biopharma 4.0 era.

New technologies in the digital factory enhance quality, efficiency, and flexibility for bio/pharmaceutical manufacturing.

Eli Lilly and Company experts share the vision and the value of the digital plant for pharmaceutical manufacturing.

System connectivity and data analysis drive increased productivity in pharma manufacturing.

Valitacell and Solentim, have announced a collaboration aimed at the acceleration of discovery and development of biologic drugs and advanced cell therapies.

Spectroscopic-based control methods were introduced as equivalent alternative methods, first to a gas chromatographic method to monitor an in-process solvent exchange step and second to a potentiometric titration method to release a process input material for drug substance manufacturing.

Technical advances in process understanding and control must be accompanied by a change in mindset.
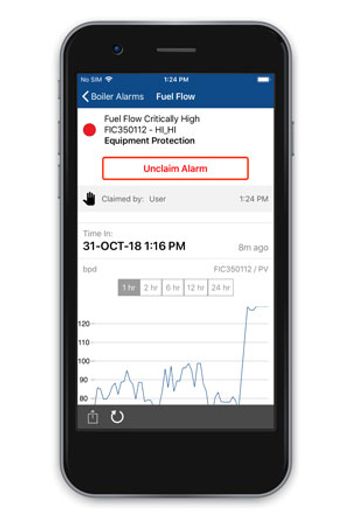
New alert customization and expanded Open Platform Communication support in Emerson’s DeltaV Mobile app improve notification and third-party system monitoring.

Tornado Spectral Systems, winner of the 2018 CPhI Excellence in Pharma Award for Analysis, Testing, and Quality Control, discusses real-time process measurement for biopharmaceutical and small-molecule drug manufacturing.