
The Natoli AIM Data Acquisition and Analytical Software for Natoli’s NP-RD10A single-station tablet press speeds research and development.

The Natoli AIM Data Acquisition and Analytical Software for Natoli’s NP-RD10A single-station tablet press speeds research and development.
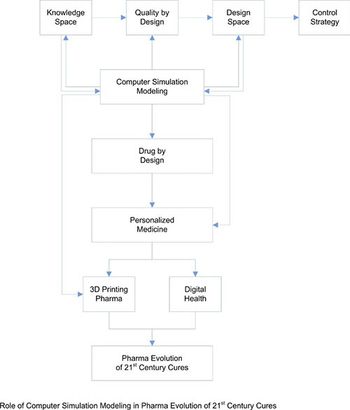
Twenty-first century drug development challenges sponsors and contract partners to collaborate more closely, using tools such as model-based simulation.

Can an Irish analytics company and its CEO bring pharma closer to 21st-century practice?

The Honeywell Connected Plant Asset Performance Insight helps manage maintenance and operations by connecting assets and equipment to the cloud and applying analytical models to avoid unplanned downtime.

Lonza Pharma & Biotech's's Modular Automated Sampling Technology (MAST) platform, which allows for the collection of up to 10 sterile sample sources for automated analysis in multiple analytical devices, won the CPhI Pharma Award 2017 for Excellence in Pharma: Bioprocessing.

The Industrial Internet of Things and new tools such as smart glasses can improve training and daily tasks for pharmaceutical manufacturing operations.

The IFPAC annual meeting on advancing the understanding and control of manufacturing processes using process analytical technology will be held Feb. 11-14, 2018.

The Industrial Internet of Things can be used in the bio/pharmaceutical industry to monitor equipment health, optimize processes, and enable modular facilities.

BioTek Instruments has released a second edition of its BioSpa software that now offers users a simplified but effective interface for kinetic imaging or detection workflows.

The Endress+Hauser iTHERM TrustSens hygienic RTD self-calibrates to verify in-line that the sensor is working as designed

A survey by LNS Research and sponsored by Honeywell showed that industrial companies are not moving quickly to adopt cyber security; calls on CEOs to take action.
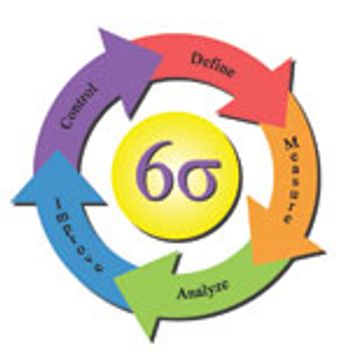
Instead of rigidly applying statistical tools, experts suggest that pharma embrace statistical thinking, but focus on reducing variability and adding value for patients.

Lawrence Yu, deputy director of the Office of Pharmaceutical Quality (OPQ), Center for Drug Evaluation and Research (CDER), and his FDA colleague, Michael Kopcha highlight the importance of Six Sigma approaches to continuous improvement in the pharmaceutical industry.

As cost pressures mount as a result of multiple biologics being developed for the same indication, manufacturers can harness process efficiencies to maintain the value of legacy products.

The process control and automation requirements of single-use systems differ from those of stainless-steel equipment.

Advances in process analytical technology have been achieved, but significant challenges remain.

Soft sensors are powerful tools that can be used along with spectroscopic instruments in on-line measurement.

A venture between GEA and Siemens aims to familiarize more pharmaceutical companies with more modern control and continuous processing.

Process analytical technology, based on monitoring particle size distribution and tracking coating thickness measurements in real time, can be used to predict the dissolution of polymer-coated multiparticulates.

An influx of millennial workers may have an impact on whether pharma manufacturers choose to implement IIoT technology.

Banner Engineering’s Sure Cross U-GAGE K50U Ultrasonic Sensor is used for wireless tank monitoring.

Industry experts discuss IIoT and its impact on pharmaceutical manufacturing.

Although best practices are key, advances in integrated informatics platforms and automation can make it easier to ensure data integrity and improve overall lab efficiency.

Improved process analytical technology and new ways of thinking seek to enhance measurement and control for next-generation pharmaceutical manufacturing.

PAT, quality by design, process controls, and analytical advances for small- and large-molecule drugs are on agenda for IFPAC 2017.