
PAT has aided formulation, both pre-and post-filing, by reducing costs and time frames.

PAT has aided formulation, both pre-and post-filing, by reducing costs and time frames.


A new release of FactoryTalk Batch software from Rockwell Automation eases recipe creation and adds security.

Siegfried Schmitt, Principal Consultant, PAREXEL International, discusses the benefits of automated processes.
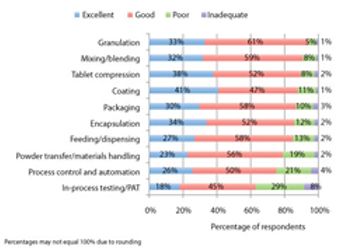
Pharmaceutical Technology’s survey collected industry feedback on trends and the utility of equipment used in finished drug-product manufacturing.
PharmTech's annual equipment and manufacturing survey shows gains in process analytical technology and indicates areas for future innovation.

The Modular Automated Sampling Technology platform allows sampling of bioreactors.
Common challenges and key considerations when developing a freeze-drying cycle for protein pharmaceuticals.

A new process analytical technology based on impedance spectroscopy has potential applications for characterizing product attributes during the freeze-drying process.
Quality-by-design principles enhance a thorough understanding of both product and process technology, which is needed for optimization of solid-dosage manufacturing, including processes for improving solubility, such as hot-melt extrusion, softgels, and liquid-filled capsules.

Automation System Improves Operational Insight

An updated bioreactor based on rocking technology has more advanced controls but is easier for biopharmaceutical manufacturers to use.
Case studies on the manufacture of a bluk powder and the development of a tablet show the application of QbD principles.

New product reviews for May 2013, featuring automation, IT, and process control systems.

A Pharmaceutical Technology survey shows satisfaction with utility and innovation in most solid dosage and parenteral drug-manufacturing equipment.

Industry is moving toward closed-loop control of continuous processing.

Process analytical technology (PAT) is being successfully used to improve understanding and optimize pharmaceutical unit operations, but greater value can be obtained by integrating PAT with overall process control of a continuous manufacturing system. Pharmaceutical Technology spoke with Ivo Backx, manager of business and project development for the pharmaceutical industry at Siemens Industry Automation Division, to gain insight on the issues involved.

Applying quality-by-design and process analytical technology facilitates process understanding and control of various operations in lyophilization.

QbD paradigm advances process understanding in development and manufacturing.

New product reviews for March 2013, featuring automation, IT, and process control systems.

New product reviews for December 2012.
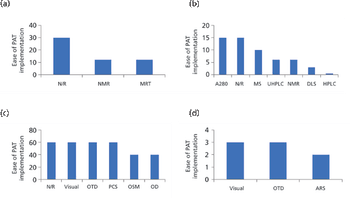
In this paper, the authors review the various analytical methods that can enable use of PAT.

Real-time experimentation may offer continuous process improvement.

Industry experts share perspectives on analytical instrumentation, methods, and data analysis.

The International Society of Automation’s (ISA’s) 7th Marketing and Sales Summit, held Aug. 15–17, 2012 in Austin, Texas, was themed “New Rules of Customer Engagement: Riding the Winds of Change”, and emphasized the need to adapt to the changing needs, expectations, and behaviors of marketplace decision makers, according to an Aug. 27, 2012 press release.