Pharmaceutical Science & Technology News

Pharmaceutical Science & Technology News

Interphex provided an opportunity to examine the latest pharmaceutical packaging concepts and packaging machines.
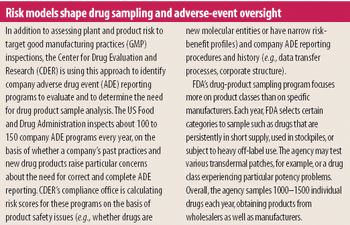
ORA leadership looks to staff redeployment and risk management to ensure product quality despite diminishing resources.

The CDER and CBER have released a new "Guidance for Industry: Q8 Pharmaceutical Development," outlining what drug manufacturers should include in the Pharmaceutical Development section of International Council on Harmonization (ICH) Common Technical Document (CTD) submissions.

Spectrum Laboratory Products (Gardena, CA) recalled its active pharmaceutical ingredient, tacrolimus, after learning that some lots of the ingredient are subpotent. At least one injury has been reported.

Research and Markets (Dublin, Ireland) has released ?Annual Investment Analysis Report of the Chinese Pharmaceutical Logistics Industry, 2005-2006.? The report provides analysis of the pharmaceutical logistics characteristics, conditions, pharmaceutical market and pharmaceutical retail dynamics.

The US Food and Drug Administration (Rockville, MD) seeks volunteers to take part in a pilot project to test a Health Level 7 (HL7) data-interchange standard for submitting product stability data.

On April 28, the US Food and Drug Administration's Center for Drug Evaluation and Research (Rockville, MD) issued a Warning Letter to Pliva Hrvatska d.o.o., a subsidiary of Pliva d.d. (Zagreb, Croatia).

The US Food and Drug Administration?s (Rockville, MD) Center for Drug Evaluation and Research (CDER) received 637 commercial investigational new drug (IND) applications in 2005, a 20-year high.

In a May 2 Federal Register notice (1), the US Food and Drug Administration withdrew its Jan. 17 direct final rule, "Current Good Manufacturing Practice Regulation and Investigational New Drugs" (2), which would have exempted manufacturing of drugs for Phase I clinical trials from most provisions of 21 CFR 211.

Sigma-Aldrich Corporation (St. Louis, MO) has acquired Iropharm, Honeywell International's custom chemical synthesis business in Arklow, Ireland. Terms of the cash purchase were not disclosed.

China's State Food and Drug Administration prepares to strengthen the enforcement of good manufacturing practices.

Animal testing and accounting can both be hazardous.

At this year's BIO conference, US Health and Human Services Secretary Mike Leavitt predicted that over the coming decade, "Medicine will be transformed from an instinctive art of alleviating symptoms to a science of personalized healthcare." Is industry ready?

Gottlieb challenges industry. First GenerationNext Awards. Implementing PAT. Optimizing site risk. RFID in the supply chain. Biodegradable polyketals. Global drug-market growth moderates.

Manufacturing and formulation innovation spurs drug develop-ment, but raises new safety and quality issues.
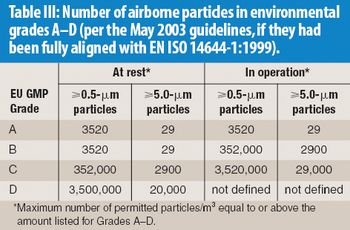
The author explores the importance of the proposals to revise Annex 1 of the EU GMPs in the context of the desire for science-based, internationally respected GMPs. Commentary also is provided about the relationship between this annex and CEN–ISO cleanroom standards.

On Feb. 21, the US Food and Drug Administration issued a warning letter to Wockhardt (Mumbai, India), a drug manufacturer. FDA cited the company for violations of CGMPs in the manufacture of drug products and active pharmaceutical ingredients.
The need to curb drug counterfeiting is spurring development of track-and-trace and product authentication technologies.

Eisai plans $105-million plant. Novartis to build plant in China. BMS building $660-million biologics facility. Slow adoption of RFID. Vaccines update. $14.7 billion in pharma construction in 2006. Chiron sells Betaseron to Schering. Baxter to develop cell based H5N1 vaccine. Drug sales up 5.4%, but growth slowing.

Guidance for reducing the risk of dissolved ozone measurement and addresses proper installation, calibration, and verification to ensure the best possible answers from dissolved ozone analysis.

Vitamin D and a sunny window shed som light on product stability.

All sectors of manufacturing are under continual pressure to bring new products to market quicker, stealing a march on the competition and maintaining their revenue stream.

India and China Position for Growth in APIs

Proposed Legislation to Help Fight Counterfeit Drugs