
FDA denies the NDA for the RA drug developed by Eli Lilly and Incyte, citing the need for more data.

FDA denies the NDA for the RA drug developed by Eli Lilly and Incyte, citing the need for more data.

Standard Homeopathic Company is recalling all lots of Hyland’s Baby Teething Tablets and Hyland’s Nighttime Baby Teething Tablets due to inconsistent amounts of belladonna alkaloids.

Pharmaceutical Technology spoke with CPhI North America presenter Jonathan Helfgott to discuss navigating GDUFA and helpful tips for submitting successful ANDAs.

The agency cited the company’s India facility for batch failures and data integrity problems.

The new guide offers guidance on how to ensure data and records are complete, consistent, secure, accurate, and available throughout their lifecycle.

A drop in US drug approvals was noted but this trend was not yet seen in Europe.

The agency released guidance on single assessments of PSURs to improve safety and benefit-risk assessment of medicines.

The role of patient advocates in shaping regulations and policy has put attention on financial and operational links between drug companies and independent health organizations.

A new study in NEJM compares the regulatory review processes of FDA and EMA.
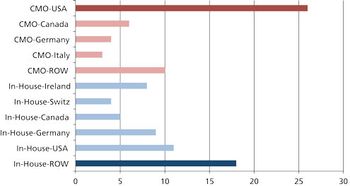
What are the pros, cons, and practicalities of moving pharma manufacturing back to the US? \

Scott Gottlieb answers Senators' questions at his confirmation hearing before the Senate Health, Education, Labor and Pensions Committee.

Deutetrabenazine is the first deuterated product approved by FDA, approval represents the first new treatment option for chorea associated with Huntington’s disease in nearly a decade.

EMA has developed a framework and action plan to foster relationships with the academic community.

Both the European Union and United States are still ironing out issues such as confidentiality of information and recognition of competence of each party’s regulatory authorities in their agreement on GMP inspections.

GW Pharmaceuticals plans to submit a regulatory filing to FDA and EMA following two positive Phase III trials of Epidiolex in patients with Lennox-Gastaut Syndrome.

The authors present the results of a survey of small- and large-molecule pharmaceutical and biopharmaceutical companies on implementation of Analytical quality by design concepts.

Susan Schniepp, distinguished fellow at Regulatory Compliance Associates, discusses the value of internal audits and how the information gained can be applied.

Industry fears limited benefits as FDA readies voluntary data tracking program.

The complex nature of biologics adds additional CQAs that must be determined to ensure the safe development of biologics
As regulators strive for balance in cGMPs for cell, gene, and tissue therapies, risk-management principles must guide decisions involving process media and additives.

Continuous manufacturing will not work for all pharmaceuticals, but the right infrastructure, senior management support, and planning from the earliest stages of drug development could eventually allow up to 80-90% of small-molecule APIs to be made continuously, says Paul Sharratt, head of process science and modeling at Singapore’s Institute of Chemical and Engineering Sciences.

The mAb is the first approved treatment that targets the progressive form of the disease.

The agency sent a warning letter to Opto-Pharm Pte Ltd. after deficiencies in sterile manufacturing procedures were found at the company’s Singapore facility.

Manufacturers and regulators are working to reach consensus on the harmonization of management of postapproval changes.

The agency recommended six drugs for approval in March 2017 including treatments for neuroblastoma, heart failure, and more.