
The global excipients market shows moderate growth, increased consolidation, and expansion activity in emerging markets and select product areas.
Patricia Van Arnum was executive editor of Pharmaceutical Technology.

The global excipients market shows moderate growth, increased consolidation, and expansion activity in emerging markets and select product areas.

Novartis presented an update on its long-term strategy, performance, and growth outlook last week, which revealed that the company is on track to deliver the strategic priorities it had set out in 2010.

Sweden's Medical Products Agency issues a report calling for strengthening environmental standards for pharmaceutical production within the European Union.

Sanofi outlined its long-term financial objectives this week as the company discussed its strategy in mitigating generic-drug incursion and in generating growth in key therapeutic areas and emerging markets.

Industry experts discuss the challenges and solutions needed to ensure security in the pharmaceutical supply chain.

Internal and external Web-based communities are changing how pharma companies can innovate.

International Federation of Pharmaceutical Manufacturers and Associations takes global action to improve public health.

Industry buy-in is increasing as pharma companies proceed with select projects and research.

Researchers at MIT and Harvard University report on new methods for producing microscale hydrogels.

Biocatalysis, chemocatalysis, and other chiral technologies continue to attract the investment dollars of CMOs and fine-chemical companies.

As contract manufacturers and fine-chemical suppliers gather for CPhI/ICSE, effective strategies for technology differentiation are key in an increasingly competitive environment.

To further enhance its position in clinical-trial materials supply, Catalent Pharma Solutions has agreed to acquire the clinical-trial supplies business of Aptuit for $410 million on a cash and debt-fee basis.

The second quarter of 2011 presented a mixed picture of financing for the life-science sector, which includes the biotechnology and medical-device industries.

The Society for Chemical Manufacturers & Affiliates commented on EPA's modifications to the Inventory Update Reporting rule, also broadly know as the Chemical Data Reporting rule. EPA issued the final rule earlier this month.

GlaxoSmithKline is among the companies building scientific capacity to address diseases in the developing world.

EPA's Presidential Green Chemistry Awards recognize developments in green chemistry, including those in pharmaceutical applications.

Merck & Co. announced last week that it plans to reduce its global workforce, as measured by year-end 2009 levels, by an additional 12–13% by 2015. The company made the announcement as part of its second-quarter earnings release, which the company issued on July 29, 2011.

Pharmaceutical companies and their suppliers share approaches for innovation in sourcing and procurement.

Xavier University, in partnership with FDA, will hold a conference in October to focus on the changing regulatory, business, and operational issues affecting the global pharmaceutical supply chain.

The US Agency for International Development, the government of Norway, the Bill and Melinda Gates Foundation, Grand Challenges Canada, and The World Bank partner to bring innovative solutions to improve maternal and children’s health in the developing world.

The pharmaceutical majors continue rationalizing manufacturing capacity in established markets as they forge their manufacturing networks in biologics and emerging markets.

O-arylation and O-alkylation, a one-pot protein synthesis, a combined approach in continued and chemocatalysis, and green-chemistry applications are the target of some recent advances in API synthesis.

O-arylation and O-alkylation, a one-pot protein synthesis, a combined approach in continued and chemocatalysis, and green-chemistry applications are the target of some recent advances in API synthesis.
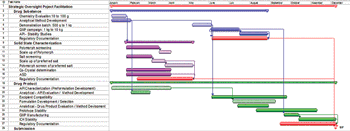
The article examines a cross-functional supplier integration model to facilitate project management.

Supplier differentiation is increasingly important in the highly competitive arena of pharmaceutical outsourcing.
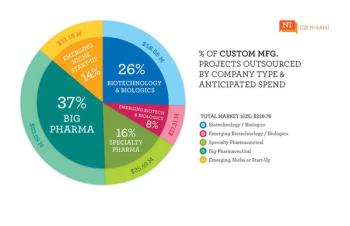
The article examines the drivers of customer perception of contract service providers of pharmaceutical development and manufacturing.

The DHS announced it has revised tiering assignments for several chemical facilities covered under DHS's Chemical Facility Anti-Terrorism Standards program, which requires chemical companies to develop and implement specific security plans for their facilities.

SOCMA has issued its support this week of the passage of pending free-trade agreements with South Korea, Panama, and Colombia by two Congressional committees.

With a new North American headquarters and expanded operations, Almac is furthering its transatlantic approach to contract development and manufacturing.

SOCMA President and CEO Lawrence D. Sloan discusses the key policy initiatives and activities of the association.