
Approaches centre on ways to optimise process conditions and operability.
Patricia Van Arnum was executive editor of Pharmaceutical Technology.

Approaches centre on ways to optimise process conditions and operability.

Al podcast with Robert Farra, president and COO of MicroCHIPS, who discusses wirelessly controlled and programmable microchip-based drug delivery as an alternative to subcutaneous injections.

Nanolipogels for anticancer drug delivery, inhalable and thermo-responsive, fat-encased nanoparticles for targeted drug delivery, and calcium carbonate microspheres are some recent developments.

US spending on prescription drugs will increase through 2016 but at historically low levels as emerging markets are projected for double-digit growth.

Abbott forms a partnership with the US Secretary of State's International Fund for Women and Girls to support grassroots organizations that are working to advance health, education, and economic opportunity for women in Burma.

What will be the future of pharmaceutical outsourcing? What factors will influence the relationships between sponsor companies and contract service providers and what practical solutions are both seeking?

MedImmune, the global biologics arm of AstraZeneca, announced that it is restructuring its infectious disease and vaccines R&D and operations. This will result in the closure of MedImmune's sites in Mountain View and Santa Clara, California.

Highlights of UN Conference on Sustainable Development, held in Rio de Janeiro, Brazil, last month and related industry initiatives.

Sponsor companies' increasing focus on strategic outsourcing has changed the rules of the game.
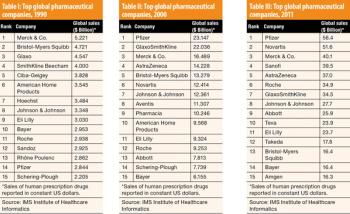
Tracking change from spinoffs of chemical companies to life-sciences powerhouses.

Flow chemistry and microreactors offer an alternative to traditional batch manufacturing.

How will the pharmaceutical company of 2020 be different from the pharmaceutical company of 2010? A look at emerging business models and the factors influencing the direction of the global pharmaceutical industry.

Biosimilars represent an emerging niche in the biopharmaceutical market, but how strong is their true potential?

Jim Miller, president of PharmSource, examines the future direction of CROs/CMOs and the factors influencing the pharmaceutical contract services sectors.

Tracking changes from spinoffs of chemical companies to life-sciences powerhouses.
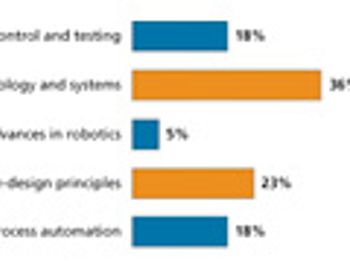
Readers point to quality by design as having a significant influence on manufacturing and drug development during the past decade.

Companies roll out expansions in manufacturing high-potency APIs and finished products.

Sponsor companies' increasing focus on strategic outsourcing has changed the rules of the game.

Advances in targeted drug delivery and customized release profiles are key goals.

Flow chemistry and microreactors offer alternatives to traditional batch manufacturing.

Bristol-Myers Squibb has agreed to acquire the biopharmaceutical company Amylin Pharmaceuticals for a purchase price of $5.3 billion and an additional $1.7 billion for assuming Amylin's net debt and a contractual payment obligation to Eli Lilly based on Amylin's recently terminated agreement with Eli Lilly over the diabetes drug exenatide.

Gold nanoparticles for targeting tumor sites and elastic capsules using nanosized flakes are some recent approaches used to control and target drug delivery.

Industry experts share perspectives on analytical instrumentation, methods, and data analysis.

Novartis, GlaxoSmithKline, and Lonza are among those participating in newly formed consortiums for developing and producing medical countermeasures.

Improving bioavailability of poorly water-soluble drugs is an ongoing challenge for formulation scientists and is of crucial importance to advance clinically promising drug candidates.

The key activity of the pharmaceutical majors influencing drug development and manufacturing thus far in 2012.

Needle-free jet injection systems, gold-coated nanoparticles, and elastic capsules with nanosized flakes advance targeted drug delivery.

Venture-capital funding is up in 2012, but public financing remains sluggish in the biopharmaceutical industry.

Advances in palladium-catalyzed hydrogenation, visible-light photocatalysis, and chemocatalyisis for heterocycles are some recent developments.

The pharmaceutical industry weighs in on WHO global health initiatives, including R&D for diseases of the developing world, a global vaccine action plan, neglected tropical diseases, and counterfeit medicines.