
Biopharmaceutical executives share their perspective on the key issues influencing growth and innovation.
Patricia Van Arnum was executive editor of Pharmaceutical Technology.

Biopharmaceutical executives share their perspective on the key issues influencing growth and innovation.

Ben Venue Laboratories is extending its voluntary suspension of manufacturing at its Bedford, Ohio, facility. The company had originally announced the suspension on Nov. 19, 2011, and announced the extension of the suspension on Dec. 23, 2011.

Some recent advances involve strategies for accelerating reaction discovery, approaches for inducing chirality and stereochemical analysis, and applications in nanotechnology for protein elucidation.

Nanosized systems are important in drug delivery. Such nanosized systems include liposomes, nanocrystals, micelles, colloidal particles, quantum dots, and dendrimers. Dendrimers are class of synthetic macromolecules with highly branched, monodispersed, circular, and symmetrical architecture that are used as carrier molecules in drug delivery.
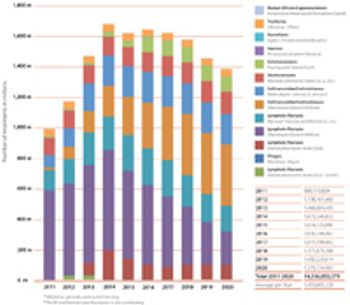
Pharmaceutical companies pledge donations of 14 billion treatments for the next decade to combat nine neglected tropical diseases.

The Generic Pharmaceutical Association has proposed a multistakeholder initiative to minimize current and future critical drug shortages. The announcement follows a series of Congressional, executive, and industry response to address the recent problem of drug shortages.

Samsung and Biogen Idec agreed to invest $300 million to establish a joint venture to develop, manufacture, and market biosimilars. The deal is Samsung's latest effort to strengthen its position in biosimilars.

International efforts, including improving access to medicines, have contributed to stemming the rise of HIV/AIDS in the developing world.

A roundup of developments in corporate social responsibility and sustainability from the bio/pharmaceutical industry, its suppliers, and other private and public organizations.

A podcast moderated by Patricia Van Arnum, executive editor of Pharmaceutical Technology.
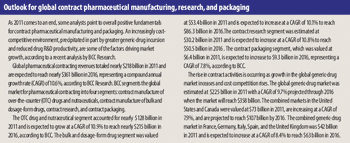
Expansion activity was limited as fine-chemical producers and CMOs of API and intermediates grapple with changing industry fundamentals.

Drug shortages, supply-chain security, generic-drug incursion, and flexible manufacturing models are some of the issues shaping the bio/pharma industry.

As 2011 comes to a close, a new paradigm of product development is ever more important.

The Society of Chemical Manufacturers and Affiliates issued a statement to the US Senate Committee on Environment and Public Works supporting the evaluation of national chemical-control laws but specifically opposing the Safe Chemicals Act, which was introduced earlier this year by Sen. Frank Lautenberg (D-NJ).

Merck & Co. highlighted the key developments in its late-stage pipeline and its near-term plans for regulatory filings. The company has 19 candidates in Phase III clinical trials and plans to submit eight new US regulatory filings in 2012–2013, including five new drug candidates.

The Society of Chemical Manufacturers and Affiliates issued a statement to support a recently introduced bill that it says will allow the federal government to more effectively deal with market-access barriers.

Pharmaceutical companies join international efforts to reduce maternal and child mortality in the developing world.

John Kelly, vice-president of strategy and transitioning sites for Pfizer Global Supply, discusses the company's manufacturing and supply strategy and network.

Pharmaceutical Technology's podcast series from CPhI.

A roundup of news from fine-chemical suppliers, CDMOs, and CMOs from this year's installment of CPhI Worldwide.

Researchers develop various catalytic approaches for improving yield, purity, stereoselectivity, and process conditions.

Pharma companies and their suppliers are tasked with managing an evermore complex clinical-trial material supply chain.

The US Senate and House of Representatives passed last week three separate free-trade pacts with South Korea, Colombia, and Panama. The Society of Chemical Manufacturers and Affiliates and the Pharmaceutical Research and Manufacturers of America both issued statements expressing support for the legislation.

AstraZeneca is investing $200 million in a new manufacturing site in China. The new site represents AstraZeneca's largest investment in a single manufacturing facility globally.

The International Federation of Pharmaceutical Manufacturers and Associations offers an action plan and research for addressing noncommunicable diseases.

The biotechnology industry achieved aggregate profitability in 2010 and funding improved, but levels of innovation capital still lag.

Industry experts share perspectives on the changing fundamentals and business models for outsourcing pharmaceutical chemical development and manufacturing

The United Nations calls for a multistakeholder plan by 2013 to curb the rise of noncommunicable diseases.

John Kelly, vice-president of strategy and transitioning sites for Pfizer Global Supply, discusses the company's manufacturing and supply strategy and network.

The global excipients market shows moderate growth, increased consolidation, and expansion activity in emerging markets and select product areas.