
Dr. Reddy's Laboratories (Hyderabad, Andhra Pradesh, India) has entered into a technology transfer, manufacturing, and marketing agreement with R-Pharm (Moscow), a Russian pharmaceutical company.
Patricia Van Arnum was executive editor of Pharmaceutical Technology.

Dr. Reddy's Laboratories (Hyderabad, Andhra Pradesh, India) has entered into a technology transfer, manufacturing, and marketing agreement with R-Pharm (Moscow), a Russian pharmaceutical company.

The European Medicines Agency outlined it work program and budget for 2011 and anticipates similar work loads for drug applications and practically no increases to its budget for that work program in 2011.

Novartis (Basel) signed a memorandum of understanding with the City of St. Petersburg, Russia, confirming its intent to build a new full-scale pharmaceutical manufacturing plant in St. Petersburg.

McNeil Consumer Healthcare, a division of McNEIL-PPC, part of Johnson & Johnson (J&J, New Brunswick, NJ) issued a voluntary recall on Dec. 9, 2010, for Rolaid products in the United States and Canada due to reports of possible product contamination.

This week, another pharmaceutical executive has stepped down, Elmar Schnee, head of the pharmaceutical division of Merck KGaA (Darmstadt, Germany).

Pfizer (New York) appointed Ian C. Read, currently head of the company's global biopharmaceutical operations, as president, chief executive officer, and director. He succeeds Jeffrey B. Kindler, who retired from the company.

PricewaterhouseCoopers (PwC) released the results of a survey that analyzed the relationship of life-science companies with the US Food and Drug Administration and their views of the agency.
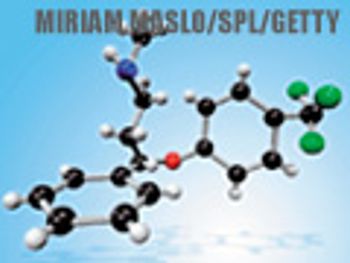
Approaches in cyclization, palladium-catalyzed cross couplings, fluorination, and natural product synthesis help to optimize routes for select drugs.

GlaxoSmithKline (GKS, London) outlined a plan to invest EUR 500 million ($649 million) in research and development (R&D) and related manufacturing in the United Kingdom conditioned on the successful implementation of a so-called "patent box" measure by the UK government.

Richard Spoor, senior vice-president of global procurement at Merck & Co., discusses the company's global procurement strategy and philosophy.

Measuring the total cost of ownership in customer/supplier relations is crucial in evaluating the performance of a procurement organization.

Pharmaceuticals, improvement in healthcare delivery systems, veterinary care, and poverty-alleviation remedies are part of an overall strategy for addressing neglected tropical diseases.

Sponsor companies need to consider regional- specific factors in the growing and changing market for contract research when outsourcing to China.

Developing and implementing true collaboration in customer/supplier relationships. This article is part of a special section on sourcing and corporate social responsibility.

Bayer (Leverkusen, Germany) announced last week a restructuring program and a strategy to strengthen its focus in research, development, and marketing of new products in its healthcare and crop-sciences businesses as well an interest in expanding its activities in emerging markets.

The International Conference on Harmonization (ICH) Steering Committee (SC) and its working groups met in Fukuoka, Japan Nov. 6-11, 2010.

The European Medicines Agency and the Massachusetts Institute of Technology's (MIT's) Center for Biomedical Innovation (CBI) and Center for International Studies (CIS) are launching a collaborative research project with a focus on enhancing regulatory science in pharmaceuticals, according to an EMA press release.

The biotechnology company Biogen Idec (Weston, MA) announced last week a major restructuring program that will refocus the company's research and development (R&D) programs, consolidate facilities, and reduce its workforce.

The Global Reporting Initiative begins an effort to increase the level and strength of sustainability reporting among US-based corporations.

Contract manufacturing organizations and fine-chemical suppliers unveiled capacity, product, and service expansions at CPhI Worldwide in Paris last month.

Pfizer (New York) announced on Oct. 29, 2010 that it intends to recall two additional lots--approximately 38,000 bottles--of Lipitor (atorvastatin calcium) 40 mg tablets distributed in the United States.

Abuse-deterrent combination drugs represent a niche area in formulation development.

An analysis of the approaches and tools used to tackle the problem of poorly soluble drugs.

Industrial and academic partnerships forge new territory in solid-state chemistry.

Puerto Rico's Governor Luis Fortuno unveiled a fiscal reform package in Puerto Rico this week as part of economic development and budget-deficit reduction plan for the commonwealth.

Pfizer announced this his week that it had entered into a strategic global agreement for the worldwide commercialization with the Indian biotechnology company Biocon (Bangalore) for biosimilar versions of insulin and insulin analog products (e.g., recombinant human insulin, glargine, aspart, and lispro).

Gregg Brandyberry, CEO of Wildfire Commerce, senior advisor of AT Kearney procurement and analytic solutions, and former vice-president of procurement of global systems and operations for GlaxoSmithKline, discusses collaborative innovation in customer-supplier relations.

An annual analysis rates the performance of pharmaceutical and biotechnology companies in corporate sustainability.

World leaders gathered at the United Nations last month to review the progress and map out a strategy for achieving the Millennium Development Goals.

A recently released industry guide outlines a science- and risk-based approach to control the risk of cross-contamination.