
Excipient manufacturers expand production capacity and partner to broaden their offerings.
Patricia Van Arnum was executive editor of Pharmaceutical Technology.

Excipient manufacturers expand production capacity and partner to broaden their offerings.
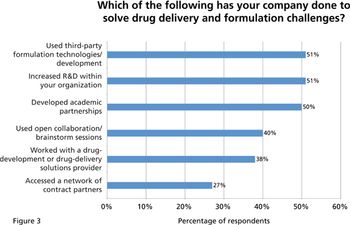
A recent survey examines the industry's views on the chief challenges and technologies in drug delivery and formulation development.

GlaxoSmithKline plans to invest more than £500 million ($798 million) in the United Kingdom across its manufacturing sites to increase production of key active ingredients for its pharmaceutical products and vaccines. The company announced selection of Ulverston in Cumbria as the location for the first new GSK manufacturing facility to be built in the UK for almost 40 years.

Unite, Britain and Ireland's largest trade union with 1.5 million members, said it will fight the closure of Sanofi's pharmaceutical manufacturing facility in Newcastle-upon-Tyne in northeast England. Last week, Sanofi announced the closure of the facility, which employs 450 people and makes solid-dose oral drugs mainly for the UK and European markets.

The Bulk Pharmaceuticals Task Force, an affiliate of the Society of Chemical Manufacturers and Affiliates (SOCMA), issued its support for the introduction of a House bill, the Generic Drug and Biosimilar User Fee Act (HR 3988), which SOCMA says will help to achieve parity between foreign and domestic firms.

Joan Connolly, president of the Drug, Chemical, and Associated Technologies Association (DCAT) and principal at Connovan Consulting, examines the key issues affecting sourcing, procurement, and the pharmaceutical supply chain.

Several major pharmaceutical companies detail their contributions for combating neglected tropical diseases.

The latest developments from contract API manufacturers and fine-chemical suppliers from Informex, held in New Orleans last month.

Nanosupensions are among the ways formulation scientists seek to address the problem of solubility.

Regulatory bodies, standard-setting organizations, and industry seek to tackle the problem of counterfeit drugs and securing the flow of pharma ingredients.

Wirelessly controlled microchips may offer an alternative to injection-based drug delivery

The article examines some recent developments for this process step and for continuous manufacturing overall.
Pharmaceutical companies, equipment providers, contract-service providers, and excipient manufacturers apply various approaches for improving solubility. The article examines some recent developments.

Taste-masking is an important consideration to ensure patient compliance.

Regulatory bodies, standard-setting organizations, and industry seek to tackle the problem of counterfeit drugs and securing the flow of pharma ingredients.

GlaxoSmithKline is recalling 394,230 bottles containing its antihypertensive drug DynaCirc CR (isradipine) controlled-release tablets. The lots are being recalled due to concerns regarding the level of cGMP compliance and procedural controls related to line clearance during the packing process at the Lincoln, Nebraska, facility of Novartis Consumer Health, where the product is made.

Last week, FDA Commissioner Margaret A. Hamburg testified before the House Committee on Energy and Commerce, Subcommittee on Health to support the fifth authorization of the Prescription Drug User Fee Act, also known as PDUFA V. She also outlined a series of recommendations, sent to Congress in January 2012, to include in PDUFA V.

Last week, FDA Commissioner Margaret A. Hamburg testified before the House Committee on Energy and Commerce, Subcommittee on Health to outline the agency’s case for supporting the fifth authorization of the Prescription Drug User Fee Act (PDUFA), also known as PDUFA V.

As biopharmaceutical development and commercialization increases, companies are expanding their cold-chain capabilities.

A new class of nanoparticles hold promise for preventing premature drug release and offering greater accuracy and effectiveness in drug delivery.

The US Department of Justice, on behalf of FDA, filed a consent decree of permanent injunction against the generic-drug manufacturer Ranbaxy in the US District Court of Maryland. The decree was filed on Jan. 25, 2012, and is subject to court approval.

A technical forum featuring Catalent Pharma Solutions, SAFC, and Neuland Laboratories.

Venture-capital financing was up last year in the US biotechnology industry, but public-equity financing, exclusive of debt, declined.

Improving R&D productivity, life-cycle management, and operational efficiency are key approaches for enhancing growth prospects.
The author examines recent examples of preferred-provider collaborations.

The author examines the opportunities and positioning of contract service providers.

Personalized medicine, which targets individualized treatment and care based on personal and genetic variations, holds much promise for the pharmaceutical industry.

FDA issued last week its recommendations for three user-fee programs: the fifth authorization of the Prescription Drug User Fee Act (PDUFA) and new user-fee programs for human generic drugs and biosimilar biological products.

The market for contract API manufacturing and fine chemicals is forecast to be fairly strong, and growth for generic APIs is expected to outpace growth for innovator APIs.

The pharmaceutical majors advance vaccines for the developing world.