
A recent poll shows the public's view of the US Food and Drug Administration has improved somewhat, but better oversight of drug safety is still needed.
Patricia Van Arnum was executive editor of Pharmaceutical Technology.

A recent poll shows the public's view of the US Food and Drug Administration has improved somewhat, but better oversight of drug safety is still needed.

Best practices show the value of effective project management when outsourcing between a virtual pharmaceutical company and a contract manufacturing organization.

Strong growth in biopharmaceuticals bodes well for contract manufacturing, but the perils and the promises of pipelines remain.

Taiwan sees to bring its intellectual property laws and enforcment into better alignment with international standards.
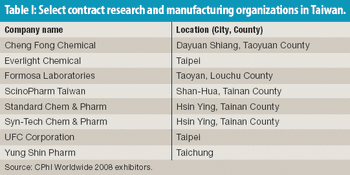
Taking a cue from its electronics industry, Taiwan is seeking to put its biotechnology and pharmaceutical industries on the map. An interactive map shows pharmaceutical activity in Taiwan.
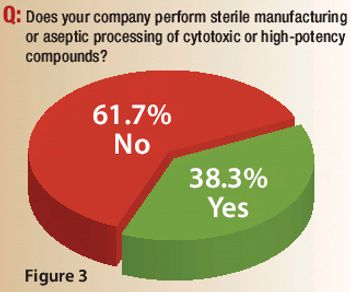
A Pharmaceutical Technology survey examines capacity expansions, outsourcing practices, innovation levels, and the role of quality by design in sterile manufacturing and aseptic processing.

The United States Pharmacopeial (USP) Convention signed a memorandum of understanding (MOU) with the Federal Service on Surveillance in Healthcare and Social Development of the Russian Federation (Roszdravnadzor) last week in Moscow.

Pfizer outlined the progress of its late-stage pipeline last week and announced this week plans to separate its research activities into two organizations, one focused on small molecules and the other on biologics, pending the closure of its pending acquisition with Wyeth.

Following several years of debate, the Senate Judiciary Committee voted 15?4 last week to approve a comprise patent reform bill, the "Patent Reform Act of 2009" (S. 515).

The financial crisis is adding further injury to already weakened prospects for the pharmaceutical industry.

Follow-on biologics or biosimilars offer a niche growth market for the pharmaceutical industry. As the process for establishing a regulatory pathway for biosimilars is debated, companies, including Big Pharma, are positioning themselves to gain this piece of the pharmaceutical pie.

The US Senate introduced a bipartisan bill (S. 726) on Mar. 26, 2009, which establishes a regulatory pathway for approval of biosimilars.

A review of recent product innovations, policy developments, and growth prospects in the excipients market.

The financial and economic downturn is likey to have long-term implications for outsourcing.

A bipartisan bill that would establish a regulatory pathway for the approval of biosimilars was introduced into the US House of Representatives last week.

The Synthetic Organic Chemical Manufacturers Association (SOCMA) said last week that Congress is likely to the include inherently safe technology (IST) measures in proposed chemical site-security legislation that is likely to be introduced in late winter or early spring.

Weakening fundamentals in 2008 and projected for 2009 dampen the outlook for pharmaceutical chemical outsourcing, but near-term growth and investments levels in R&D and capital spending remain fairly robust.

Gilles Cottier, SAFC's new president, plans to continue a growth strategy that emphasizes value-added technologies positions, growth in Asia, and customization in its supply-chain solutions.

FDA issued a notice in the Federal Register last week requesting comments on the automated collection of four additional pieces of information that are not available in the US Customs and Border Protection's (CBP) data set.

Contract manufacturers of APIs and intermediates report gains, but express caution.

Contract manufacturers deploy a business model using operations in the US and Western Europe with facilities in Asia.
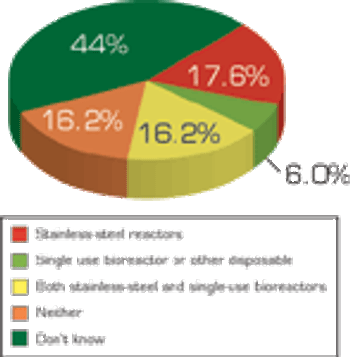
Pharmaceutical Technology's annual survey on equipment and machinery shows fewer companies increased spending in 2008 and still fewer will increase spending in 2009 as overall economic conditions affects purchasing decisions.

After a year of increased attention on the pharmaceutical supply chain in Asia, what will be the region?s short? and long?term role?

The United States Trade Representative (USTR) is seeking documentation from chemical companies to identify possible non-tariff trade barriers, created by the European Union's Registration, Evaluation, Authorization and restriction of Chemicals (REACH) regulation, which would be inconsistent with the EU international trade obligations under World Trade Organization (WTO) rules, according to an informational release by the Synthetic Organic Chemical Manufacturers Association (SOCMA).

Sanofi Aventis CEO Chris Viehbacher provided the company's growth strategy in light of changing conditions facing the pharmaceutical industry, which include patent expirations and declining research and development (R&D) productivity.

The US Food and Drug Administration sent letters to manufacturers of certain opioid drug products, indicating that these drugs will be required to have a Risk Evaluation and Mitigation Strategy (REMS).

The US Food and Drug Administration issued its first approval for a biological product produced by genetically engineered animals.

The Federal Trade Commission has filed a complaint in federal district court challenging agreements in which Solvay Pharmaceuticals (Marietta, GA) paid generic drug makers Watson Pharmaceuticals (Corona, CA) and Par Pharmaceutical Companies (Woodcliff Lake, NJ) to delay generic competition to Solvay's branded testosterone-replacement drug "AndroGel," a prescription pharmaceutical with annual sales of more than $400 million, according to an FTC press release.

Cambrex CEO Steve Klosk looks to higher valued-added segments such as high-potency actives, controlled substances, and drug-delivery technologies to drive growth.

Several contract manufacturers announced capacity and service expansions at InformEx, the exhibition of custom and batch manufacturers, held in San Francisco, last week.