
Buoyed by increased demand for pharmaceutical outsourcing services, Ricerca Biosciences proceeds with an expansion plan.
Patricia Van Arnum was executive editor of Pharmaceutical Technology.

Buoyed by increased demand for pharmaceutical outsourcing services, Ricerca Biosciences proceeds with an expansion plan.

Emerging pharmaceutical companies represent an important client base for CROs and CMOs. Lessons learned for successful customer–supplier relations.

SAFC, Regis Technologies, Carbogen Amcis, Cambridge Major Laboratories, and AMRI are among the companies at CPhI Worldwide in Frankfurt this week that are moving forward with expansions.

The biopharmaceutical company ImClone Systems rejected Bristol-Myers Squibb's previously announced bid of $60 per share in cash or $4.5 billion for acquiring ImClone, citing the bid as "inadequate."

The European Chemical Industry Council and five national chemical associations representing France, Germany, Italy, Spain, and the United Kingdom, have launched ReachLink. ReachLink is a company founded to help companies participate in the Substance Information Exchange Forum (SIEF), which is designed as an information-sharing vehicle to facilitate companies in meeting requirements under REACH

Boehringer Ingelheim and and Hisoar (Taizhou, China), a pharmaceutical production company, formed a strategic production alliance in China.

The US Food and Drug Administration will hold a public meeting this month to gain public input on implementing the recommendations of the Nanotechnology Task Force report, taking another step closer to setting a regulatory framework for nanotechnology for pharmaceuticals and medical devices.

The pharmaceutical majors deploy green-chemistry strategies to improve the synthesis of active pharmaceutical ingredients and intermediates.

Sterile manufacturing may be the next aspect of pharmaceutical manufacturing to consider in the continuous process paradigm.

The selection of the chiral stationary phase is an important consideration in separating enantiomers when using high-performance liquid chromatography, supercritical fluid chromatography, and simulated moving bed chromatography.

Fueled by a need to reduce costs and improve efficiencies, continuous processing may be the next paradigm shift in pharmaceutical manufacturing.
Carbon–hydrogen functionalization, ketone α-alkylation, and biocatalysis are some recent advances in asymmetric synthesis.

The outlook is fairly optimistic as contract manufacturing organizations (CMOs) gather at CPhI Worldwide in Frankfurt. CMOs are expanding capacity for small-molecules, biologics, and finished-product manufacturing.
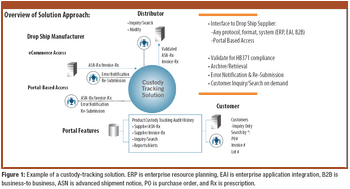
EPedigree, track-and-trace technologies, and other tools for optimizing supply-chain management are of increasing importance to the pharmaceutical industry. The author examines the current regulatory and legislative framework for ePedigree for finished drug products as well as proposals to require electronic statements for pharmaceutical ingredients.

The specialty drug company King Pharmaceuticals is seeking to acquire the specialty pharmaceutical company Alpharma (Bridgewater, NJ) through an unsolicited bid of $33 per share or $1.4 billion in cash. Alpharma has rejected the bid.

The Synthetic Organic Chemical Manufacturers Association (SOCMA) is expressing trade concerns with the European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation, a new European Union policy on chemicals and their safe use.

Actavis Totowa LLC, the US subsidiary of the generic drug manufacturer Actavis Group, is announcing a voluntary recall to the retail level of all drug products manufactured at its Little Falls, New Jersey, facility. This is a precautionary, voluntary action by Actavis following an inspection conducted by the Food and Drug Administration earlier this year.

The House Committee on Energy and Commerce issued a revised discussion draft to the Food and Drug Administration Globalization Act of 2008.

Bristol-Myers Squibb is seeking to acquire the biopharmaceutical company ImClone Systems (New York) for $4.5 billion or $60 per share. BMS currently holds a 17% stake in ImClone.

The pharmaceutical industry?s outsourcing practices receive increased attention as Congress evaluates the role of outsourcing in drug-safety issues.

Strong profitability and good growth prospects bode well for the global market for contract research organizations.

CMOs expand capacity and capabilities in high-potency manufacturing to meet strong demand for cytotoxic and other potent drugs.

This year's meeting of the Controlled Release Society unveiled a plethora of research insights.
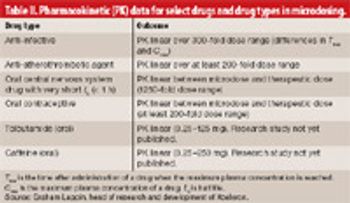
J. Scott Tarrant, executive vice-president of Xceleron, explains the role of microdosing in drug development. He describes how microdose data can be used to predict pharmacological dose absorption, distribution, metabolism, and excretion/pharmacokinetic outcomes using accelerator mass spectrometry.

Gonghua Pan, associate director and head of the parallel medicinal chemistry sourcing operations at Pfizer, explains the evolution of the company's approach to outsourcing research and development from a line-or function-centric approach to an integrated sourcing model. This analysis includes the role that contract research organizations in Asia play in the company's outsourcing actvities.
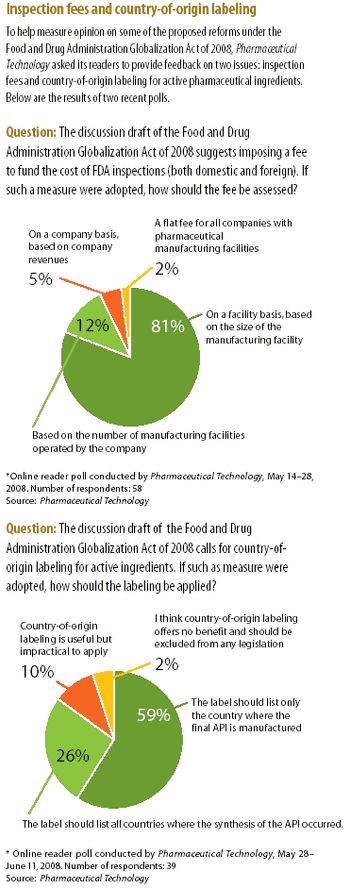
The high-profile case of contaminated heparin from a Chinese supplier has intensified the debate on the effectiveness of FDA's process for inspecting foreign drug-manufacturing facilities. The article examines proposed legislative and regulatory reforms and actions taken by the agency to improve drug-import safety.

As part of a new strategic direction, GlaxoSmithKline CEO Andrew Witty outlined three priorities that are designed to improve the company's long-term financial performance.

Roche plans to acquire the biopharmaceutical company Genentech (South San Francisco, CA) for $43.7 billion. Roche currently holds a 55.9% stake in Genentech and it plans to acquire the remaining publicly held minority interest that it does not own for $89 per share.

3M Drug Delivery Systems has successfully designed a proof of concept device using a solid microstructured transdermal system for the systemic delivery of high-potency pharmaceuticals. The technology was showcased at a poster session at the annual meeting of the Controlled Release Society held this week in New York City.

Pfizer has agreed to pay $975,000 in civil penalties to resolve alleged violations of the Clean Air Act at its former manufacturing plant in Groton, Connecticut, according to a release by the US Department of Justice.