
A recent US government study shows that follow-on biologics are likely to have a favorable economic impact if proposed legislation authorizing a regulatory pathway in the US is approved.
Patricia Van Arnum was executive editor of Pharmaceutical Technology.

A recent US government study shows that follow-on biologics are likely to have a favorable economic impact if proposed legislation authorizing a regulatory pathway in the US is approved.

Richard E. Maleczka Jr. and Milton R. Smith III, professors of the Department of Chemistry at Michigan State University, were recognized by the Environmental Protection Agency's Presidential Green Chemistry Challenge Awards, for designing a catalytic reaction to synthesize precursors for the Suzuki-coupling reaction.

A roundtable with John Doney, Jiao Yang, Hans Baer, and Elena Draganoiu.

Natale S. Ricciardi, president of Pfizer Global Manufacturing and senior vice-president of Pfizer, discusses the company's new manufacturing focus that features plant network optimization, increased outsourcing, and greater adoption of agile or lean manufacturing.
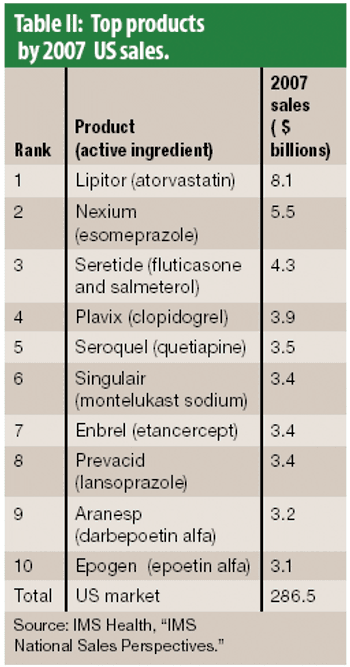
The year 2007 was slow for approvals for new molecular entities and overall pharmaceutical industry growth. Big Pharma seeks relief in a growing biologics portfolio.
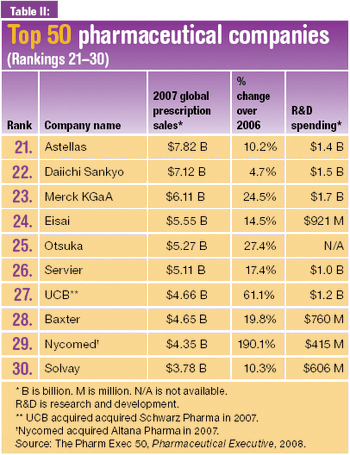
The pharmaceutical majors invest in biologics production capacity as they advance restructuring programs and build their pipelines

Bayer and its subsidiary Icon Genetics (Halle, Germany) have developed a production process to produce therapeutic proteins from tobacco plants.

Senator Sherrod Brown (D-Ohio), member of the Senate Health, Education and Labor Pensions (HELP) Committee, is requesting additional information from the US Food and Drug Administration and the pharmaceutical industry, concerning the regulation and practice of outsourcing.

The European Chemicals Agency (ECHA), the European Union regulatory body overseeing the implementation of the REACH (Registration, Evaluation, and Restriction of Chemicals) regulation, began full operations on June 1.

The Synthetic Organic Chemical Manufacturers Association is evaluating proposed legislation, The Climate Security Act of 2008 (S. 3036), which directs the Environmental Protection Agency to establish a program for decreasing greenhouse emissions.

The global and US biotechnology industries performed well in 2007 as each recorded higher revenues and inched toward aggregate profitability.

As part of a larger effort to address drug counterfeiting, the European Commission is seeking to tighten manufacturing and related supply-chain requirements for active pharmaceutical ingredients.

Antibody drug conjugates offer a niche opportunity in drug development and contract manufacturing.

The US Senate approved a measure (HR 2642) that would provide the US Food and Drug Administration with $275 million in additional funding under a supplemental appropriations bill. The measure now goes before the House.

The European Federation of Pharmaceutical Industries and Associations (EFPIA), the trade association representing European pharmaceutical manufacturers, issued recommendations to the public consultation launched in March by the European Commission's proposed drug anticounterfeiting measures. EFPIA's proposal includes a ban on drug repackaging.

Eli Lilly completed the final and largest phase of a major biotechnology expansion in Indianapolis, Indiana.

Increased investment by the pharmaceutical and biotechnology majors in offshore early drug development creates opportunities and challenges for outsourcing.

Congress is considering proposing a new chemical policy similar to the European Union’s REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation. Batch and custom manufacturers weigh in on the debate.

Congressional hearings were held last week on the Food and Drug Administration Globalization Act discussion draft.

Florida is making its case for pharmaceutical and biotechnology research and development.
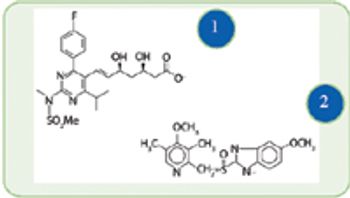
Chemocatalytic and biocatalytic routes show promise for more efficient syntheses of select active ingredients.

Congress is taking on the issue of inspection of foreign drug-manufacturing facilities as it began hearings on a discussion draft of the Food and Drug Administration Act of 2008.

The US House of Representatives Energy and Commerce Committee is proposing legislation to address the funding and authority of the US Food and Drug Administration in regulating the safety of the country's drug supply.

Ell Lilly plans to streamline a portion of its manufacturing operations in Indianapolis.

Takeda Pharmaceutical agreed to acquire Millennium Pharmaceuticals for $8.8 billion in cash or $25 per share.

Schering-Plough announced a major new productivity transformation program with a goal of achieving $1.5 billion in annual savings.

US pharmaceutical and biotechnology research companies invested $58.8 billion in research and development in 2007, according to analyses by the Pharmaceutical Research and Manufacturers of America and Burrill & Company.

The former formulation-development business of MDS Pharma Services seeks to build its niche in early-phase drug development.

The economic case for personalized medicine offers a solution to the industry's recent woes.
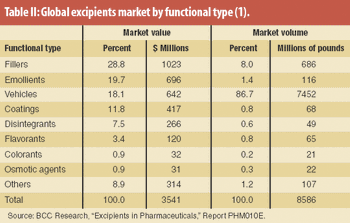
Moderate growth is projected for the global excipients market. Excipient producers target blends and new grades for improving functionality and performance.