
Financial experts share their insights for the performance for innovator drug companies and generic-drug players.
Patricia Van Arnum was executive editor of Pharmaceutical Technology.

Financial experts share their insights for the performance for innovator drug companies and generic-drug players.

Excipient producers and industry observers share their perspectives on innovation for excipients.

The US market for prescription pharmaceutical grow only 3.8% in 2007, the lowest growth rate in more than 40 years, according to a recent analysis by IMS Health.

The US Pharmacopeial Convention announced a revised glycerin monograph in the United States Pharmacopeia.

The US House of Representatives Committee on Homeland Security approved legislation last week that mandates inherently safer technologies (IST) as part of chemical-site security standards, a move that was opposed by the Synthetic Organic Chemical Manufacturers Association.

Merck & Co. advised AstraZeneca (London) that it will not exercise its option to sell its interest in certain AstraZeneca non-proton pump inhibitor (non-PPI) products this year.

Leveraging its global infrastructure, SAFC Supply Solution pilots a new external sourcing program.

Draft federal legislation that would require high-risk chemical facilities to use inherently safer technology for reducing their risk may present potential problems for custom and batch manufacturers supplying the pharmaceutical industry.
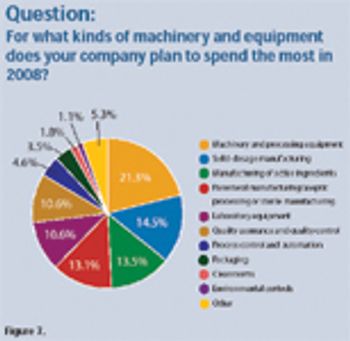
The pharmaceutical industry plans moderate increases in spending for equipment and machinery in 2008. Investments include equipment for solid-dosage manufacturing, active pharmaceutical ingredients, and parenteral manufacturing.

Contract manufacturers of APIs and intermediates are cautiously optimistic.

SAFC is delivering on its plan for double-digit annual growth by increasing its businesses through organic growth and targeted technology acquisitions.
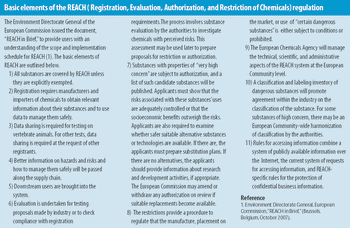
The European Union's REACH initiative has the potential to affect the flow of chemicals into the pharmaceutical suppy chain.
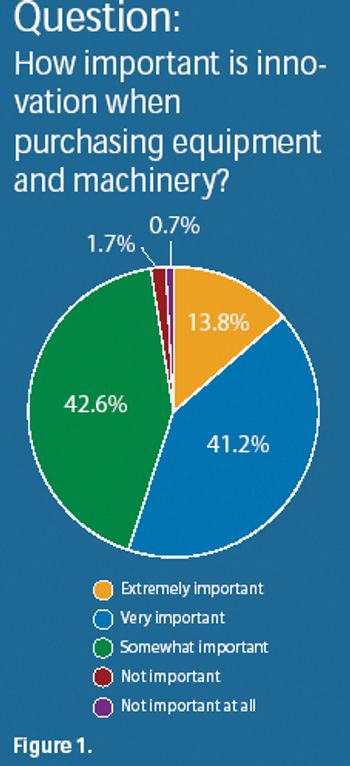
Results from Pharmaceutical Technology's Equipment and Machinery Trends survey and industry members provide insight into product innovation

A preview of some product enhancements and launches for Interphex 2008, the large trade show being held Mar. 26–28 in Philadelphia.

The US Patent and Trademark Office rejected the patentability of claims of a patent by Genentech that related to certain methods used to make antibodies and antibody fragments by recombinant DNA.

Baxter Healthcare Corporation is providing an update to its January 2008 heparin sodium injection 1000 units/mL 10 and 30mL multi-dose vial voluntary recall of nine lots, which the company initiated as a precautionary measure due to an increase in reports of adverse reactions that may be associated with the drug.

Contract manufacturers of active pharmaceutical ingredients and intermediates unveil expansion plans and strategies at this year's Informex.

A recent business outlook survey conducted by the Synthetic Organic Chemical Manufacturers Association reveals a generally favorable view of current and future business conditions for contract manufacturing of active pharmaceutical ingredients and intermediates.

Contract manufacturers expand capabilities in aseptic processing, clinical-trial materials supply, and cytotoxic manufacturing.

Merck & Co. and Pfizer signed separate pacts for preclinical drug candidates to treat schizophrenia.

Richard Spoor, senior vice-president of global procurement at Merck & Co., Inc., discusses the company's strategy and progress made in its supply strategy that involves increased outsourcing and implementing lean-manufacturing principles in its manufacturing network. Pharmaceutical Technology's senior editor Patricia Van Arnum moderates.

The National Nanotechnology Initiative's new strategic plan affirms the federal government's support for research and development in nanotechnology, including for medical and healthcare applications.
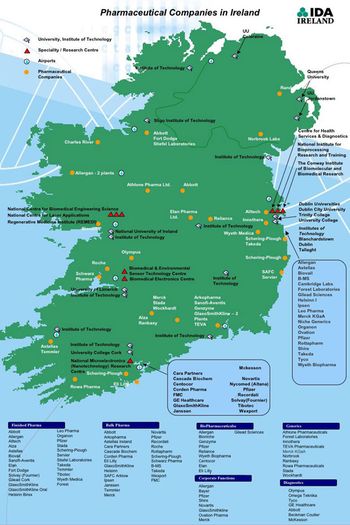
Ireland seeks to maintain a leading position in pharmaceutical and biopharmaceutical manufacturing as it also builds its base in research.

Singapore moves forward with a plan to diversify its life science investment with projects in biologics and drug discovery.
Puerto Rico seeks to build its standing in biopharmaceutical manufacturing and research as it retains its role in bulk pharmaceutical and dosage manufacturing for small molecules.
Researchers and process chemists share approaches in synthesis of active ingredients and pharmaceutical intermediates.

The US Food and Drug Administration's joint panel of the Nonprescription Drugs Advisory Committee and the Endocrinologic and Metabolic Drugs Advisory Committee voted against recommending approval of the over-the-counter use of Merck & Co.'s “Mevacor” (lovastatin) 20 mg.

Merck & Co. initiated a voluntary recall of 11 lots of its Haemophilus influenzae type B vaccine, Pedvaxhib, and two lots of its combination Haemophilus influenzae type B/ hepatitis B vaccine, Comvax.

The US Food and Drug Administration issued a rule that clarifies its requirements for current good manufacturing practices for aseptic processing, water standards, and verifications standards.

The scientific demands on the US Food and Drug Administration far exceed its capacity to respond, according to a recent report by the Subcommittee on Science and Technology of the FDA Science Board.