
The pending merger of Pfizer and Wyeth provides Pfizer with greater strategic diversification, particularly in biologics, but only minimally addresses its generics exposure in the near term.
Patricia Van Arnum was executive editor of Pharmaceutical Technology.

The pending merger of Pfizer and Wyeth provides Pfizer with greater strategic diversification, particularly in biologics, but only minimally addresses its generics exposure in the near term.
The pharmaceutical majors forward projects in biocatalysis, solvent replacement, and other approaches in green chemistry.

The US Department of Health and Human Services's Biomedical Advanced Research and Development Authority has awarded Novartis a contract valued up to $486 million over eight years to support the design, construction, validation, and licensing of a US cell-based influenza vaccine manufacturing facility in Holly Springs, North Carolina.

Frank Torti, currently principal deputy commissioner and chief scientist of the US Food and Drug Administration, will take over as FDA's acting commissioner next week, according to an official with the US Department of Health and Human Services (HHS).

The generic-drug manufacturer Actavis reached an agreement on a consent decree of permanent injunction with the US Food and Drug Administration regarding the company?s US subsidiary Actavis Totowa LLC.

Outsourcing sterile manufacturing involves an integrated approach in product life-cycle management.

Achieving double-digit growth through 2011, biotech-based active pharmaceutical ingredients (APIs) are expected to far surpass growth rates for chemically synthesized APIs.

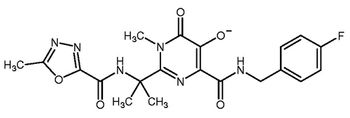
Catalysis for olefin metathesis and aldol reactions and synthetic routes to natural products are some recent gains.

After a year of increased attention on the pharmaceutical supply chain in Asia, what will be the region's short- and long-term role? This article contains bonus online-exclusive material.

The US Food and Drug Administration issued a draft guidance, Genotoxic and Carcinogenic Impurities in Drug Substances and Products: Recommended Approaches.

At its annual business briefing held last week, Merck & Co. outlined its short- and long-term strategy for growth. Its strategy is focused on increased penetration in emerging markets, the establishment of a business for developing follow-on biologics or biosimilars, and a new commercial model for product life-cycle management.

The Environmental Protection Agency is proposing to add hazardous pharmaceutical wastes to the Universal Waste Rule to provide a system for disposing of hazardous pharmaceutical wastes.

The Synthetic Chemical Organic Manufacturers Association (SOCMA) has raised concerns over requirements for security vulnerability assessments (SVA) under the US Department of Homeland Security's (DHS) Chemical Facility Anti-Terrorism Act Standards (CFATS).

Reps. John D. Dingell (D-MI), current chairman of the Committee on Energy and Commerce in the US House and Representatives, and Bart Stupak (D-MI), chairman of that committee?s Oversight and Investigations Subcommittee, said that moving the Food and Drug Administration Globalization Act and other measures for drug and food safety will be a key priority for the next Congress.

A recent study shows life-science companies have difficulty in planning and executing change-management practices.

Asian countries are moving up the value chain for pharmaceutical outsourcing.

2009 will likely be a difficult period for emerging biopharmaceutical companies.
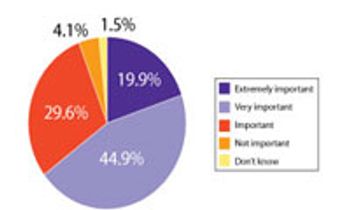
A recent Pharmaceutical Technology survey examined the level, sources, and reasons behind innovation in drug development and manufacturing. This article contains bonus online-exclusive material.

Companies at the American Association of Pharmaceutical Scieintists (AAPS) unveiled technologies, expansion plans, and services for formulation development, manufacturing, and drug delivery at the AAPS Annual Meeting and Exposition held in Atlanta last week.

The American Association of Pharmaceutical Scientists (AAPS) recognized researchers in the pharmaceutical sciences at AAPS Annual Meeting and Exposition in Atlanta last week.

Novo Nordisk will invest nearly $400 million to build a new insulin plant in Tianjin, China.

The European Medicines Agency's (EMEA) Committee for Medicinal Products for Human Use recommended that the status of GlaxoSmithKline's anti-obesity drug "Alli" (orlistat) be switched from prescription-only to nonprescription.

Near-stagnant growth for the US pharmaceutical market, the rising influence of emerging markets and specialty products, and increased penetration of generic drugs are major issues for the global pharmaceutical market.

Uncertain economic times crimp the financing flow into the US biotechnology industry.

Optimizing the solid form of a drug reaps scientific and technical awards.

Pharmaceutical Technology will feature video coverage of AAPS this month.

John Dingell (D-MI), chairman of the US House Committee on Energy and Commerce and Bart Stupak (D-MI), chairman of that committee's Subcomittee on Oversight and Investigations, directed a letter to US Food and Drug Administration Commissioner Andrew C. von Eschenbach to request further information regarding FDA's process for inspecting manufacturing facilities of the generic drug manufacturer Actavis following several product recalls by the company.

A bill that would require country-of-origin labeling for active and inactive ingredients for all prescription and over-the-counter pharmaceuticals was introduced in the US Senate last week.

The European Fine Chemicals Group (EFCG) and the International Pharmaceutical Excipents Council of Europe (IPEC Europe) announced the formation of a European Pharmaceutical Excipients Certification Project to develop advocacy and stakeholder management in Europe and to give advice to two European working teams as part of an effort to develop a certification program for manufacturers and distributors of pharmaceutical excipients.

Leading professionals join the community of experts panel of Sourcing and Management.