
AbCellera and Rallybio are teaming up to discover, develop, and commercialize novel antibody-based therapeutics for rare diseases.

AbCellera and Rallybio are teaming up to discover, develop, and commercialize novel antibody-based therapeutics for rare diseases.

Catalent is set to expand its biologics analytical services with its new facility in Durham, NC.

Emmes’ new facility is designed to support clients conducting cell and gene therapy research worldwide.

Under the collaboration between Vertex and Entrada, the companies will work on discovering and developing intracellular endosomal escape vehicle therapeutics for myotonic dystrophy type 1.

Thermo Fisher Scientific’s new facility in Hangzhou, China, is designed to boost biologics and sterile development and manufacturing capabilities in the Asia-Pacific region.

The objectives of the new opportunity are to improve the quality and/or use of RWD, promote better understanding of RWE study designs, and develop specific tools to evaluate aspects of RWE generation.

Amgen’s $27.8 billion acquisition of Horizon Therapeutics, an Irish biotechnology company, is one of the largest of 2022.

Takeda will purchase the rights to Nimbus Pharmaceuticals candidate, NDI-034858, which is currently being evaluated for treating multiple autoimmune diseases.

The approval is based on results from the pivotal EFFISAYIL 1 Phase II clinical trial.

mRNA-1273.222 has also received FDA EUA for children and adolescents between six and 17 years of age and for adults over the age of 18 years of age.

Generate Biomedicines published a preprint describing technology that can generate novel proteins towards desired functional or other properties.

PictorLabs, a digital pathology company developing an AI-powered virtual staining platform, formally announced its launch at the start of December.

The guidance discusses statistical approaches for BE comparisons and focuses on how to use these approaches both generally and in specific situations.

The R&D analytical solutions will consist of analytical lab services that use USP’s in-house scientific expertise and state-of-the-art facilities at the USP Advanced Manufacturing Technology Lab in Richmond and the USP headquarters facility in Rockville, Md.

Ferring Pharmaceuticals’ Rebyota was approved for the prevention of recurrence of Clostridioides difficile infection.

The partnership will see the pharma giants partner with researchers from A*STAR and various Singapore universities.

In addition, personnel, equipment, and facilities from the acquired companies will expand Reaction Biology’s presence in Germany while enabling its global customers to leverage Bioassay’s expansive portfolio of regulated clinical and commercial services.

The ROSS Vertical Blender enables mixing of friable solids.

Steraline’s VFCM100 guarantees sterility throughout the aseptic filling process via a double-wall isolator.

ACP Systems’ quattroClean snow jet technology uses liquid, climate-neutral CO2 for cleanroom cleaning.
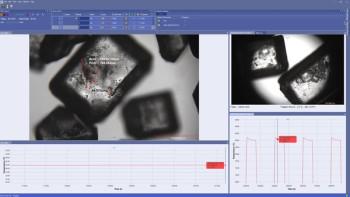
Linkam Scientific Instruments’ NEXUS software enables holistic environmental controls in experiments.

Catalent has completed the expansion of its clinical supply facility located in Shanghai, China.

Results from Eisai and Biogen’s Alzheimer's treatment, lecanemab, indicated significantly slowed cognitive decline in patients relative to placebo.

Merck will use BigHat’s AI/machine learning-enabled platform to design candidates for up to three drug discovery programs.

AstraZeneca inked a deal worth up to $320 million to purchase Neogene Therapeutics, a biotech start-up focused on T-cell receptor therapies.

The agreement will see Immutep and Merck KGaA, Darmstadt, Germany jointly fund the INSIGHT-005 study.

Aptamer’s new 18,000 ft² facility will expand the company's capacity to deliver novel binders for bioprocessing, diagnostic, and drug development.

Etihad Cargo is one of the only airlines to hold IATA CEIV Pharma certification globally.

The decision to move forward in Phase III development stems from the threat of resistance to current malaria treatment growth.

SPX-001, a respiratory drug candidate, will be submitted through a combined Clinical Trial and Ethics Committee application for further advancement in the clinical stages.