Drugmakers seeking to block the activity of a protein may have a new strategy at their disposal.

Drugmakers seeking to block the activity of a protein may have a new strategy at their disposal.

Staff reductions are not surprising with the state of the economy and falling pharmaceutical sales.

This review article explains how self-emulsifying drug delivery systems can increase the solubility and bioavailability of poorly soluble drug.

A new book explains process analytical technology, drug stability, and quality.
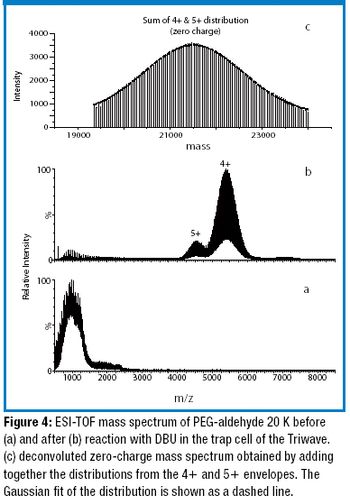
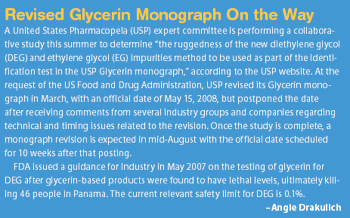
The authors developed a method to accurately measure the average molecular weight of large poly(ethylene glycols) (PEGs) using ion-mobility time-of-flight mass spectrometry coupled with gas-phase ion–molecule reactions.

A roundtable with John Doney, Jiao Yang, Hans Baer, and Elena Draganoiu.

The year 2011 may seem far off, but there is much to do to prepare for electronic pedigrees.

The authors describe the factors affecting reconstitution time of dry powder for injection and classifies them as intrinsic and extrinsic parameters.

A growing preference for public-private partnerships is spurring innovation and technology among Dutch pharmaceutical companies.

Natale S. Ricciardi, president of Pfizer Global Manufacturing and senior vice-president of Pfizer, discusses the company's new manufacturing focus that features plant network optimization, increased outsourcing, and greater adoption of agile or lean manufacturing.

Clear labels for substances that can be used as excipients, APIs, or both are critical to end-product use.

They may have seemed harmless at first, but these ideas took a big bite.

Forums, blogs, and more can spark innovation.

Polyethylene glycol (PEG) conjugation is a highly effective technical and commercial strategy to develop macromolecules. The authors explain the benefits and process of PEGylation and how it may be applied to small molecules.

With no economic relief in sight, industry, like all of us, is grappling with high-and new-costs.

New scientific discoveries promise to expand treatments for rare and neglected diseases.

Providing the right information upfront may ease new requirements to assess solvent levels.
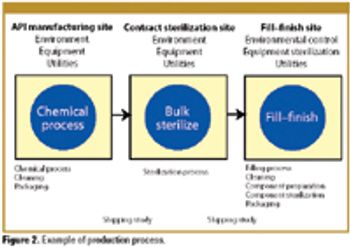
Patient safety must be the primary concern of any validation effort. The author explains how a risk-based approach to validation and compliance follows naturally from this premise.
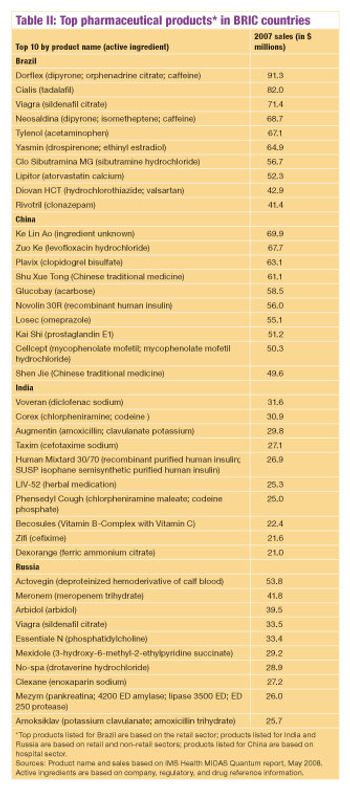
Recent reports discuss the future of pharma's emerging markets.

Editors' Picks of Pharmaceutical Science & Technology Innovations
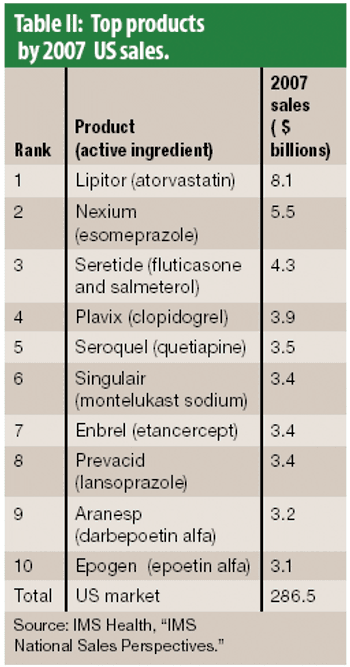
The year 2007 was slow for approvals for new molecular entities and overall pharmaceutical industry growth. Big Pharma seeks relief in a growing biologics portfolio.
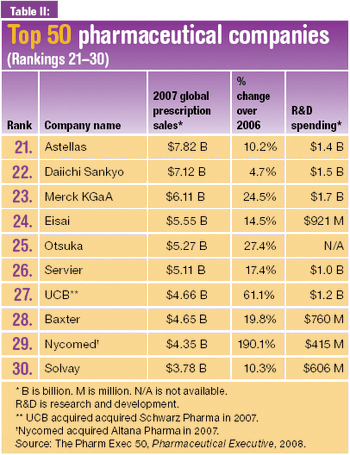
The pharmaceutical majors invest in biologics production capacity as they advance restructuring programs and build their pipelines