
The Ross VSL-50 is a portable 50-gallon agitated vessel with silicone heating blankets and temperature sensors fabricated in stainless steel 316 and designed for atmospheric operation.

The Ross VSL-50 is a portable 50-gallon agitated vessel with silicone heating blankets and temperature sensors fabricated in stainless steel 316 and designed for atmospheric operation.

A risk-based approach is recommended for analytical method comparability for HPLC assay and impurities methods.

Demand for new therapies and vaccines spotlights production challenges.

The recovery of an occasional mold does not merit any particular concern. On the other hand, evidence of mold proliferation indicative of infection of facilities or equipment must be taken seriously and requires the prompt implementation of corrective and preventive actions.

Romaco Kilian's KTP 420X tablet press compresses up to 360,000 tablets an hour.

GEA Pharma Systems' Buck MC valves are designed to address powder-handling needs for solid dosage form production.

Temperature-controlled packaging trends include prequalified systems that simplify adoption, reusable systems that are more sustainable, and new temperature-monitoring technology.
Safer fluorinating reagents and access to GMP fluorination capabilities remain challenges in API synthesis.

Fette Compacting America FE75 Tablet Press is designed to produce up to 1.6 million tablets per hour.

Data analytic strategies can help companies capitalize on personalized medicine.

AAPS supports graduate-level programs impacted by cutbacks in funding and resources.

Weighing the pros and cons of hot-melt extrusion and spray drying.
Review challenges in the use of normality testing situations and recommendations on how to assess data distributions in the pharmaceutical development manufacturing environment

Regulatory agencies in Europe are working to harmonize the marketing approval pathway of generic medicines.

Filtration system allows scalability
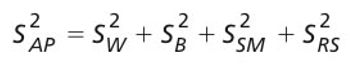
A risk-based guard band surrounds a specification limit and is derived from the uncertainty of the reportable value of the analytical procedure, which includes the uncertainty in the reference standard. The author discusses requirements for generating a reportable value and calculating the associated measurement uncertainty.

Brazil's pharmaceutical industry is optimistic, but is the pharmaceutical market growing steadily or showing signs of instability?

Siegfried Schmitt, Principal Consultant, PAREXEL International, discusses the benefits of automated processes.

Advances in solid and liquid formulation techniques are providing more options.
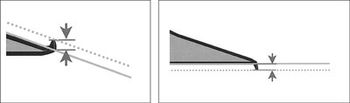
To achieve the high quality standards required for critical defects in pharmaceutical glass syringes, a combination of visual and camera-based inspection technologies are used.

The trend of exits from the CMO industry looks to be gaining momentum.

Click the title above to open the Pharmaceutical Technology October 2014 issue in an interactive PDF format.