
Oxford Genetics received £1.61 million from Innovate UK to explore computational and synthetic biology approaches for optimized mammalian bioproduction.

Oxford Genetics received £1.61 million from Innovate UK to explore computational and synthetic biology approaches for optimized mammalian bioproduction.

IPEC-Americas held elections for executive committee positions at the annual board of trustee’s dinner.

GlaxoSmithKline opened a new vaccines R&D center in Rockville, MD creating up to 200 new jobs.

DuPont received a grant from the Bill & Melinda Gates Foundation to produce pharmaceutical proteins.

BMS changes its US geographic footprint with R&D investments and closures.

Saneca Pharma has received a EUR1.5 million grant from the Slovak Ministry of Education, Science, Research, and Sport, which will be used to drive forward new R&D initiatives for API manufacturing.

Congress enacted the 21st Century Cures legislation, which shores FDA operations and supports biomedical research at the National Institutes of Health.

At the American Society of Hematology Annual Meeting, Novartis and Kite Pharma both released results from early-phase clinical trials with CAR-T therapies.

Spark Therapeutics and Selecta entered a license agreement for Selecta’s gene-therapy co-administration technology.

FDA issued a warning letter to Interquim, SA for CGMP deviations at its Barcelona API facility.

Takeda and Lightstone have launched Cerevance, a neuroscience company focused on developing therapeutics for neurological and psychiatric disorders.

JHL Biotech and Sanofi collaborate on the development and commercialization of biological therapeutics in China.

Through a license agreement with the Broad Institute, Charles River Laboratories will now be offering gene-editing engineering technology.

QuintilesIMS forecasts global spending on medicine will reach $1.5 trillion by 2021.

Dara Corrigan examines the Mutual Reliance Initiative as a method for expanding FDA’s inspection capabilities in Europe and beyond.

Researchers test the efficacy of a new polymer that is an alternative to PEG for drugs used to treat type 2 diabetes.

FDA and BARDA awarded a contract to Continuus Pharmaceuticals to develop an end-to-end continuous manufacturing process for solid-dosage drugs.

Susan Schniepp, distinguished fellow at Regulatory Compliance Associates, discusses training personnel on a limited budget.

Boehringer Ingelheim and China Southeast University announced a joint research collaboration to develop new regenerative treatments for hearing loss.

The UK’s NICE recommended pembrolizumab for the treatment of patients with advanced lung cancer, reversing the institute’s earlier decision.

Recipharm and Laccure AB signed a commercial collaboration for the manufacture and delivery of Laccure’s bacterial vaginosis treatment.

The company opened a new facility in Dublin for its cell performance technology.

Vetter’s Ravensburg data processing center received certification from a German industrial testing organization, certifying that the company’s customer and process data are protected.

WMFTG acquired Aflex Hose adding polytetrafluoroethylene-lined flexible hoses to its fluid path product portfolio.

AstraZeneca entered a collaboration with Bicycle Therapeutics for the identification and development of bicyclic peptides.
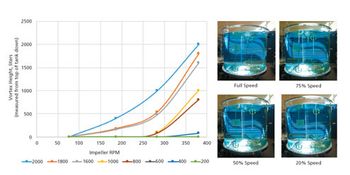
Quantitative and qualitative tools allow better understanding of mixing in a single-use bioprocessing system.

Rookie API developers beat pharma at its own game.

Europe-based bio/pharma employees are unified on satisfaction with employment conditions-and dissatisfaction with salaries.

The House-approved 21st Century Cures legislation supports initiatives for medical research, drug development, and faster approvals, but comes up short on actual appropriations.

Idifarma has acquired a Bosch GKF-702 capsule filling machine that can manufacture 3000 to 42,000 capsules per hour.