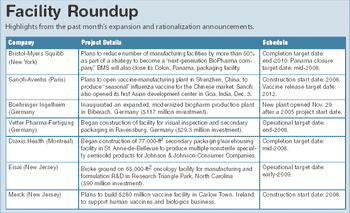
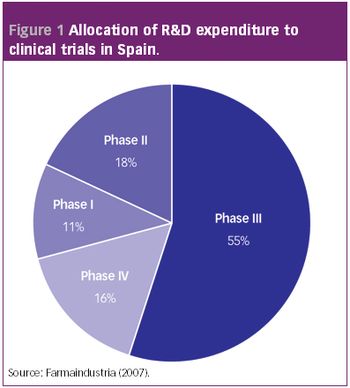
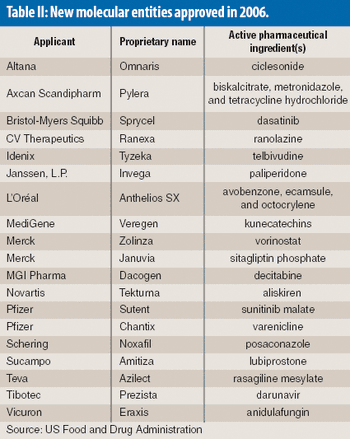
The $6.3 billion Indian pharmaceutical industry is at a crossroad. Aiming to be the international home for quality drugs, which could in itself propel India's market to $20 billion by 2015 according to recent estimates, the generic hothouse is clearly moving beyond its earlier low-cost mindset.