
The agency is expanding its use of artificial intelligence (AI) with the deployment of agentic AI for staff to create more complex AI workflows and harness AI models.

The agency is expanding its use of artificial intelligence (AI) with the deployment of agentic AI for staff to create more complex AI workflows and harness AI models.
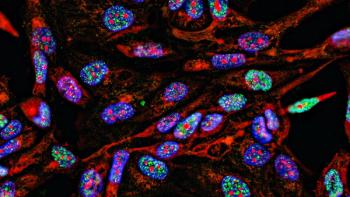
Live cell phenotypic screening can enhance translational predictability and speed up lead optimization.

As advanced manufacturing technologies reshape pharmaceutical production, the Qualified Person (QPs) must evolve from a traditional document reviewer into a digitally fluent leader capable of navigating complex data ecosystems, real-time analytics, and automated control systems. This article presents a holistic framework for “Certification by Design,” highlighting how QPs can ensure compliant, agile, and science-based batch release in the era of Industry 4.0.

The first patient visit has taken place for the SURMOUNT-REAL UK clinical trial of Eli Lilly and Company’s weight-loss drug, tirzepatide.

3D printing enables personalized precise drug delivery, enhances compounding efficiency, and is driving new regulatory models like distributed pharmaceutical manufacturing.

With big moves and investments being made via pharma outsourcing, it’s important for industry professionals to stay on top of trends and innovations.

AI transforms formulation, bioanalysis, and manufacturing; accelerated FDA reviews demand parallel development readiness; new nasal spray enhances patient drug access.

While the deadline to make oral presentations has passed, public comment can be submitted up to a month after the workshop is held on Dec. 16.

Five experts in the bio/pharmaceutical industry explore the FDA Commissioner’s National Priority Voucher (CNPV) pilot program.

Machine learning tools are now moving from concept to validated applications, driving transformative shifts in bioanalysis, preclinical testing, and formulation optimization across the industry.

Five bio/pharmaceutical industry experts discuss the impacts of the FDA Commissioner’s National Priority Voucher (CNPV) pilot program.

AI, in-silico modeling, and rational design streamline drug formulation; rapid regulatory pathways demand pre-submission perfection in quality and parallel execution.

Industry experts weigh in on the CNPV's demands for flawless CMC pre-submission, affordable pricing, expanded communication, and more.


In this episode of the Ask the Expert video series, Susan J. Schniepp, Regulatory Compliance Associates (RCA), Siegfried Schmitt, Parexel, and Anita Michaels, RCA, explain how CDMOs can best handle regulatory inspections and client expectations.

The conclusion of this interview with Sanjay Konagurthu focuses on simulations and calculations that go beyond simple screening.

FDA’s CNPV pilot aims to speed drug reviews but raises questions about safety trade-offs, resource strain, and unclear incentives for sponsors.

Sanjay Konagurthu of Thermo Fisher Scientific discusses how AI and ML can help solve the dilemma of poor solubility.

To meet rising demand, developers of precision radiopharmaceutical therapies must overcome challenges in global isotope supply and align R&D platforms with evolving FDA guidance on clinical dosimetry.

The European Union aims to become the most attractive destination for clinical research.

Mechanistic models and hypothesis-driven strategies generate optimized efficient solutions for drug development, says Catalent’s Nathan Bennette at AAPS PharmSci 360.

Quality, stability, sustainability, and the increased and thoughtful integration of artificial intelligence are foremost in the minds of those on the leading edge of testing trends.
CHMP supports an enhanced nusinersen regimen, according to Biogen, indicating possible SMA treatment shifts and international regulatory decisions.

Corey Bloom of Lonza outlines major points from his AAPS presentation, from drug delivery technology selection to the importance of CDMO partnerships.

Accelerated drug development insights using AI/digital twins, advanced bioanalysis, targeted delivery, and optimized formulation stability models.

To prevent catastrophic loss of expertise and investment, government and academic leaders say coordinated policy and significant investment in workforce talent are urgently needed in the UK.

Take our survey to voice your opinions on the bio/pharma industry impacts of the FDA Commissioner’s National Priority Vouchers program.
A worldwide, exclusive license is being granted to develop and commercialize enzyme replacement therapies using proprietary platform technology.

At AAPS PharmSci 360 2025, Elly Zhou says digital twins helped forecast the effects of drugs on human drugs via a digital control arm.

At AAPS PharmSci 360, Xialing Li, PhD, says 3D-printed budesonide tablets achieve precise, delayed delivery to the ileum for IgA nephropathy, lowering in vivo variability.