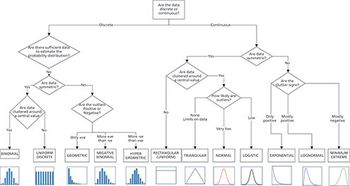
Using the central limit theorem concerning the distribution of means allows one to justify the assumption of the normal distribution.

Using the central limit theorem concerning the distribution of means allows one to justify the assumption of the normal distribution.
The author provides a direction for identifying genotoxic impurities early in the drug development process, regulating genotoxic impurities at acceptable levels in the API or drug product, and avoiding negative product regulation late in the development and/or marketing process, including expensive recalls.

Research into peginterferon alfa-2b’s degradation pathways suggest that drug substance be immediately and continuously converted to drug product when the material is in liquid form.

PPD is expanding its bioanalytical lab in Richmond, VA, to enhance its immunochemistry, biomarker, and chromatography services.

Cell DIVE is a new cell imaging technology from GE Healthcare that allows for more precise biomarker analysis.

The new microscope gives fast, deep, and clear images for live observation over long periods and allows scientists to study a larger range of applications.

Validation of analytical procedures require assessment of the impact of variations within laboratories; however, guidance to study intermediate precision has been lacking. Science and risk-based principles should be used in the design of intermediate precision studies.

Novartis revealed results from two new clinical trials indicating that Entresto (sacubitril/valsartan) significantly improves measures of cardiac structure and function in heart failure with reduced ejection fraction.

At-line NIR measurements can replace a laboratory HPLC measurement for API content in oral solid-dosage drug manufacturing.

In this interview, the main uses and benefits of NIR spectroscopy in tableting are discussed.

Regulatory bodies are taking a more stringent approach to excipient quality, but unlike guidance on GMP for APIs, those for excipients offer a framework only, meaning the onus lies with the manufacturer or supplier.

As regulatory guidance has evolved, changes in CCIT testing have also become apparent. In this article, possible CCIT strategy approaches are outlined.
The authors provide an introduction to aluminum adsorbed vaccines, review studies of antigen stability, and propose test methods for the analysis of aluminum vaccine release and stability analysis.

The editors welcome technical article contributions from the bio/pharma industry.

Protagen Protein Services’ recently acquired Brucker Tensor II IR spectrometer significantly expands the company’s analytical capabilities.

GNA Biosolutions has completed its latest round of Series C financing in which it raised US$13.5 million.

The Tridex Protein Analyzer from IDEX offers the ability to directly measure protein titer from a bioreactor in real time.

A design strategy can ensure conflicting properties are managed appropriately for multi-API controlled-release formulations.

A risk-based approach to CMC enables drug sponsors to focus on clinical and manufacturing development paths.

Pharmaceutical Technology Europe celebrates 30 years in publishing the latest insights, analysis, and developments of the bio/pharma industry.

Instrumentation advancements over the past 30 years have certainly enabled greater efficiency in pharma development and analysis despite slow adoption by the industry.

To deal with the complex requirements of biopharmaceuticals, companies need a sophisticated toolbox of analytical and purification techniques.

Machine vision systems have been an integral part of pharma manufacturing and packaging for many years and, with the introduction of stricter safety regulations, are set to become more vital.

The ZipChip CE-ESI interface was evaluated for suitability as a platform approach for quantitation of MIs in API.
Protein characterization is a critical part of drug development, but as there are still limitations with available techniques, industry needs to look at technological advances to meet the specific requirements of complex molecule characterization.