
Formulatrix’s FLO i8 and Flow Axial Seal Tip liquid handlers offer automated transferring.

Formulatrix’s FLO i8 and Flow Axial Seal Tip liquid handlers offer automated transferring.
Drug developers must understand the complex bioanalytical assays for cell- and gene-therapy drug development programs and ensure that partners have the specialized expertise needed for complex therapeutic classes.

Outsourcing of analytical testing and processes can help bio/pharmaceutical companies expand their product profiles.

Pharmaceutical Technology spoke with John Pirro, senior director of large molecule bioanalysis at WuXi AppTec, which provides anti-drug antibody (ADA) and neutralizing antibody (Nab) assay development, validation, and sample analysis services, about best practices to ensure that biologics are safe and effective.

SGS announces expansion of cell bank and bulk harvest testing services.

The company has built a fit-for-purpose liquid chromatography–mass spectrometry (LC–MS) system to streamline analytical monitoring tests for biopharmaceuticals.

Tornado Spectral Systems, winner of the 2018 CPhI Excellence in Pharma Award for Analysis, Testing, and Quality Control, discusses real-time process measurement for biopharmaceutical and small-molecule drug manufacturing.

A new plasma B cell antibody discovery workflow launched by Berkeley Lights enables the shortening of antibody drug discovery from month to a day.

The Steritest NEO device from MilliporeSigma provides safer pharmaceutical product testing through additional features.
This article demonstrates how qualitative physical attributes testing can be used to characterize soft gel capsule rupture/disintegration during rectal administration.
Early adoption of the right approach to address solubility can deliver significant benefits.

Intellectual challenge, work/life balance, compensation, and an unclear business outlook create uncertainty among European bio/pharma employees.
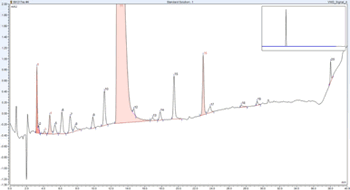
Integration of two separate chromatography data systems boosts workflow efficiency.
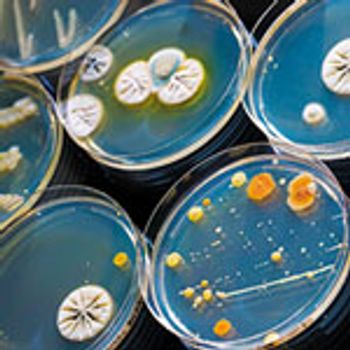
Microbial identity data can be critical for determining contamination sources.

Drawing on practical experience, the authors examine key questions and answers about various aspects relating to the enhanced approach for analytical procedure lifecycle management.
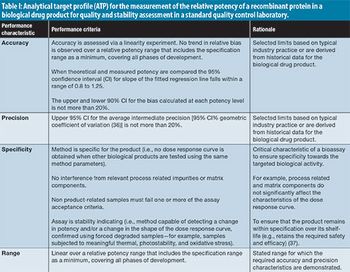
Three hypothetical analytical target profiles (ATPs) are provided, reflecting the current thinking of the the European Federation of Pharmaceutical Industries and Associations Analytical Lifecycle Management Team.

A new, high-throughput microplate reader cuts down on screening time and works faster than standard ultra-high-performance liquid chromatography processes.

The partnership, co-funded by Enterprise Ireland, will develop technologies for monitoring the quality of biopharma processes.

Industry–academia collaborations seek to address unmet needs in measurement science.

The testing of raw materials is essential as raw material quality determines the outcome of biologic product quality.

By reducing the number of assays needed and allowing product quality attributes to be measured end to end, MAM promises to allow users to gain product and process understanding much sooner than they could in the past, and to ensure quality and safety in a more efficient, streamlined way, throughout the product life cycle.
By adapting techniques from other sciences-and exploring better tools for biologics drug development-researchers are addressing challenges of protein characterization.

If a vortex mixer produces too much energy during sample preparation for particle size analysis, the size and morphology of particles can change. A study compares the applied shear to sample suspensions of ibuprofen to observe the effects of applied shear on the particle size distribution.

This article discusses why it is important to apply risk analysis, QbD, and DoE in the development of analytical methods.

This paper describes how the concept of acceptance value can be redefined to remove bias and more closely reflect quality targets.