
Lonza’s Capsugel Colorista capsule R&D solution can cut overall development time and offers greater flexibility during technical color development.

Lonza’s Capsugel Colorista capsule R&D solution can cut overall development time and offers greater flexibility during technical color development.
Integration of new modeling and analytical tools with flow chemistry are notable trends.

Efforts strive to harmonize bioanalytical method validation for non-clinical and clinical studies.

This article applies the basics of stability, performance, and capability to modern process performance and capability indices.

Experts weigh in on up-to-date analytical procedures for the lot release testing of small-molecule pharmaceuticals.

The LCMS-9030 quadrupole time-of-flight (TOF) liquid chromatograph mass spectrometer from Shimadzu is a researchgrade mass spectrometer suited to deliver high-resolution, accurate-mass detection with fast data acquisition rates.

The companies collaborated to launch a new cell-based profiling service for biochemical assay.

New software tools by Tecan, a provider of automated laboratory instruments and solutions, complement each other to enhance process monitoring of its liquid-handling platforms.

The European Medicines Agency’s detection of a second nitrosamine in a sartan API is driving a deeper dive into tetrazole chemistry; root-cause investigations will now include not only valsartan and losartan, but candesartan, irbesartan, and olmesartan.

Research by Thermo Fisher Scientific and LumaCyte suggests that LumaCyte's Radiance instrument offers the ability to rapidly analyze viral vaccines to speed development and production and ensure their effectiveness.
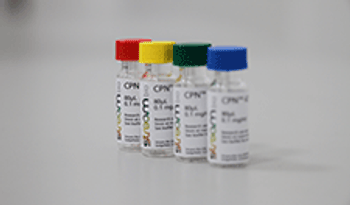
The companies have partnered to launch Conjugated Polymer Nanoparticle (CPN) products for use in molecular imaging and R&D applications.

Lonza’s new PyroTec Pro Robotic Solution provides a fully automated workflow for endotoxin detection.

As it investigates the root cause of an impurity discovered in valsartan, FDA extends its studies to APIs with similar synthesis processes.

Sharing know-how can help resolve common bio/pharma technical challenges.
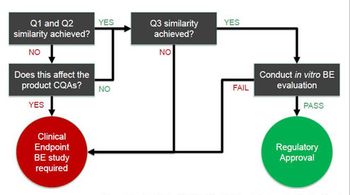
This article examines IVBE testing requirements for topical creams and explores some of the analytical techniques necessary.

Flush solutions for liquid chromatography mass spectrometry (LC-MS) systems and ultrapure solvents for ultra-high-performance LC-MS systems from Thermo Fisher Scientific can minimize interference and maximize lab instrument uptime.

Shimadzu’s Imagereveal MS mass spectrometry imaging data analysis software can analyze large sets of data or simultaneously analyze multiple sets of data.

The acquisition strengthens Nelson Labs’ outsourced testing capabilities for the pharmaceutical and medical device industries.

Protagen Protein Services, a CRO, now offers quicker and more accurate characterization of biomolecular stability using differential scanning calorimetry (DSC).

The TSQ Fortis Triple Quadrupole Mass Spectrometer from Thermo Fisher Scientific offers fast, robust liquid chromatography-mass spectrometry (LC-MS/MS) analysis for clinical research laboratories.

The Lumis electron backscatter diffraction (EBSD) detector incorporates a large-format complementary metal oxide semiconductor (CMOS) sensor to enable higher sample throughput, higher resolution measurements, and precise phase identification.

The company’s new electron microscopy and microanalysis solutions offer scientists enhanced research capabilities.

An expansion of laboratory facilities in Geneva, Switzerland expands SGS services for high-order structure analysis.

Researchers from the Department of Chemistry and Warwick Medical School developed a way to synthesize polymers to accelerate antimicrobial activity screening.
Materials in contact with a drug must be fully characterized to ensure they do not negatively affect the safety and efficacy of the product.