Eppendorf’s ViscoTip pipette tip from its Combitips advanced line can process liquids with viscosities from 200 to 14,000 mPa-s, including glycerol 99.5%, ointments, and creams.

Eppendorf’s ViscoTip pipette tip from its Combitips advanced line can process liquids with viscosities from 200 to 14,000 mPa-s, including glycerol 99.5%, ointments, and creams.

Airflow visualization studies, or smoke studies, confirm unidirectional airflow patterns in an aseptic processing facility.

Bioprocess understanding, the right equipment, and automation help, but multifunctional teamwork is the key to API production success.

The working acceptance limits for acceptance values (AV) are determined using the critical values at, for example, 95% coverage over the corresponding AV distributions. However, validity of such limits needs to be elaborated.
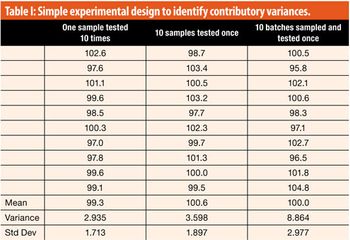
This article looks at a simple structured approach to assigning variance contributions and to assuring that the analytical procedure is fit for purpose.

Geofrey Wyatt has been named CEO and Clifford Wyatt named president of Wyatt Technology.

The company will showcase its drum-filling technology among other products and services at the upcoming ACHEMA 2018 event in Frankfurt, Germany.

The company’s investment in new laboratory equipment and personnel will strengthen its cell line development services.

The company showcased newer products and equipment for cell culture laboratories at Analytica 2018 in Munich, Germany.

Proteus Digital Health is collaborating on a pipeline of digital, oral solid-dosage drugs for various therapies, including cardiovascular and oncology drugs, based on its first NDA, which used ingestible sensors in an antipsychotic treatment.
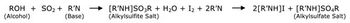
Understanding the advantages and suitability of different methods to measure residual moisture content in lyophilized materials-and the respective limitations-aids in selecting the most appropriate method for testing.

The new platform is expected to speed up cell line development.

The contract renewal allows AMRI to continue to provide medicinal chemistry and ADME services for advancing drug discovery programs in neurotherapeutics.

The expanded Laboratory Testing Division, located in Cranbury, NJ, will expand the company’s testing services in the United States.

Innomech has developed an innovative new test station for Fluidic Analytics to streamline R&D programs. The new test station serves as a labor-saving R&D tool to help process multiple test samples and to ensure product quality.

A new book by Robert Thomas, principal consultant at Scientific Solutions, provides a training tool for novices and inexperienced users of plasma spectrochemistry as well as for supervisors and senior management who want to better understand the analytical issues. Measuring Elemental Impurities in Pharmaceuticals: A Practical Guide, published on Feb.




A new scientific publication examines analytical processes for the emerging legal cannabis industry.

Access to multiple analytical techniques is essential for fully characterizing complex protein formulations.

Gilson has added Gilson Connect, a cloud-connected platform that powers a product line of Bluetooth-enabled, smart liquid-handling devices that enable scientists to achieve verifiable science.

The Concentrating Pipette Select from InnovaPrep is a small automated benchtop instrument that can concentrate contaminates from large volumes of liquids for improved detection.

Research suggests that radiation can have a significant impact on the composition and rheology of hydroxyethyl cellulose-based medicinal gels.

The company’s close communication with customers has enabled it to bring advanced pipetting products to market.