
The agreement gives IncoCell Tianjin, a wholly-owned subsidiary of China-based Boyalife Group, access to Cesca’s celluar processing contract development and manufacturing services.

The agreement gives IncoCell Tianjin, a wholly-owned subsidiary of China-based Boyalife Group, access to Cesca’s celluar processing contract development and manufacturing services.

The collaboration between the two companies aims to finish all necessary work needed to file for a first-in-human study by early 2019.

The company plans to install 4000-L disposable bioreactors from ABEC at its new commercial manufacturing facility in Wuxi city, China.

Process analytical technology and advanced process control strategies can be applied to either batch or continuous manufacturing of solid-dosage drugs.

Sartorius Stedim Biotech has launched a new automated parallel bioreactor system for perfusion culture.

The delivery device and drug form should be considered when choosing a test method for identifying and measuring particulates.

Revisions to chapters on glass containers and elastomeric closures were canceled following review of comments.

GlobalData reports the need to shift away from egg-based manufacturing of vaccines in light of influenza-related deaths.

Under this agreement, the companies will develop in parallel an antibody drug candidate and cell lines for other potential candidates.

Fluid Imaging has introduced a new imaging flow microscope at Pittcon 2018 in Orlando, FL.

The pharma major will spin out six molecules in early stage inflammation and autoimmune programs into a new company called Viela Bio.

LabVantage offers laboratory information management system solutions that include additional industry-specific functionalities.

CEM reports that the EDGE extraction system for rapid sample preparation of gas chromatography/liquid chromatography samples from CEM can prepare samples for molecular analysis within 5 min.

JLL, a real estate and investment management firm, recently released a new report on three trends shaping labs for the future of life sciences R&D.

The company showcased various updated and new analytical systems and solutions for food analysis and pharmaceutical, clinical research, and materials sciences applications.

New, user-friendly analytical systems provide high-throughput comprehensive characterization and quantitation of small and large molecules.

Shimadzu has added the compact MALDI-8020 for mass spectrometry applications.

New and emerging products advance bio/pharma laboratory operations.

Stability testing on APIs/finished drug product helps define optimal drug packaging for shelf-life storage.
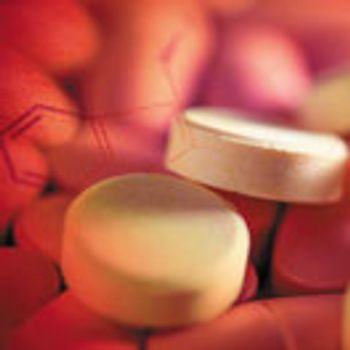
Tableting instruments (i.e., compaction simulators) that simulate high-speed presses can be used in a quality-by-design (QbD) approach to perform in-depth material characterization and direct scale-up.

Can an Irish analytics company and its CEO bring pharma closer to 21st-century practice?

The company aims to add the additional analytical services in the European Union throughout 2018.

The study suggests that circumventing evolution in cell factories can enable the commercialization of new biobased chemicals to large-scale.

Unlike current approaches where bioconjugation is typically done following the manufacture of the monoclonal antibody and the cytotoxic drug, the new method begins with the antibody supernatants and eliminates the need for extensive chromatographic purification.

A $800-million acquisition of MPI Research expands Charles River's offerings for early-stage contract research.