
A Supreme Court decision and improvements in analytical processes may speed the biosimilar approval process.

A Supreme Court decision and improvements in analytical processes may speed the biosimilar approval process.

Industry experts discuss how advances in analytical testing tools have helped address challenges in pharmaceutical analysis.

A bathless dissolution tester from Distek operates without a water bath and associated maintenance.

Real-time alternatives to dissolution testing are required for continuous manufacturing to reach mainstream use.

The more pharma science and technology change, the more business and policy concerns stay the same.

Incorporating structural constraints into pharmacokinetic studies of iron sucrose formulations.

Russell Madsen, president, The Williamsburg Group, and member of the Pharmaceutical Technology editorial advisory board, shares his thoughts on technology innovations and regulatory implications.

Cheaper drugs are good for consumers, but cost restrictions limit drug company incentives to modernize facilities.

The author reviews key technological expectations of EU GMP inspectors on the integrity of e-records.

The company added new products to its InfinityLab LC Series which will be showcased at HPLC 2017 in Prague.

Sartorius Stedim Biotech combined the company’s ambr 15 bioreactor system with the Nova BioProfile FLEX2 cell culture analyzer for laboratory experiments.
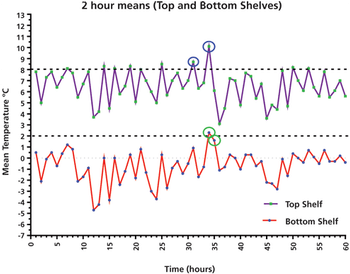
A case study presents important lessons for using average and individual results, as well as user requirements, when qualifying a laboratory refrigerator.

The past several months have seen new product releases and updates made to already available laboratory equipment.

Industry experts discuss best practices for dissolution testing of poorly soluble, immediate-release, and controlled-release formulations and the different analytical approaches used.
In this study, the authors describe a forensic microscopy approach to characterize particles that were visually observed during stressed stability testing of an ophthalmic solution formulation.

Phenomenex opened a new manufacturing and development facility in CA for the company’s gas chromatography columns.

This column presents a data case study of a laboratory refrigerator and its qualification performance over five days, with important lessons for using average and individual results, as well as user requirements.

Spectrum Pharmacy Products opens pharmacy institute for training in pharmaceutical compounding.

The pharmaceutical industry is making efforts by internally assessing, developing, and implementing semi-continuous manufacturing processes to improve manufacturing efficiencies.

Understanding the purpose of the biological indicator can guide the development of an effective sterilization process.

At CPhI North America 2017, the US Pharmacopeial Convention will be discussing its upcoming revision and modernization of the standards for elemental and organic impurities.

Innovative new technologies released over the past several months seek to enhance bio/pharmaceutical development and manufacturing.
Advances in chemical synthesis are enabling greener, more cost-efficient processes for API manufacturing.

Solid-phase extraction has several advantages over liquid/liquid extraction for extractables and leachables studies.

The FPB-1P5 sanitary lab mixer is the newest addition to Eirich Machines’ OptimaBlend Fluidizing Blender family of horizontal batch mixing systems.