
Fluence Analytics’ Argen is a protein and polymer stability monitoring product that uses continuous light scattering measurements to produce an information stream.

Fluence Analytics’ Argen is a protein and polymer stability monitoring product that uses continuous light scattering measurements to produce an information stream.

Amgen is seeking approval for an additional indication in glucocorticoid-induced osteoporosis for its blockbuster osteoporosis therapeutic, Prolia.

AstraZeneca is seeking approval for its anti-cancer monoclonal antibody, Imfinzi, for treating non-small cell lung cancer in the European Union.

BARDA has entered a $12-million contract with biopharmaceutical firm Achaogen for the late-stage development of an antibiotic against resistant bacteria, and as a potential treatment for biowarfare agents.

Johnson & Johnson’s Janssen Sciences Ireland UC will invest more than EUR 300 million (US$355 million) to expand manufacturing capacity for biologic medicines at its Ireland facility.

Clariant’s Tri-Sorb Desiccant Tablets are designed for USP moisture testing of blister packs.

Under an agreement, ProBioGen will develop a stable cell-line for and manufacture an anti-cancer antibody for Chiome using its proprietary cancer cell-killing technology.

Tools aid scale-up and comparison of single-use and stainless-steel bioreactors.

The Thermo Scientific Pharma mini implant line is an integrated solution built around the Thermo Scientific Pharma mini HME twin-screw micro compounder.
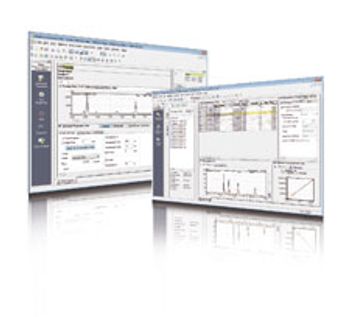
An updated version of Shimadzu’s LabSolutions analytical data system incorporates additional functions to comply with data integrity regulations, and to support development and quality inspection procedures.

Is there a difference between a specification and a standard?

The authors demonstrate that a proper measure of HPMC solution concentration is its electrical conductivity rather than its viscosity.

Amgen will collaborate with China’s Simcere Pharmaceutical Group, a R&D-driven pharmaceutical company, to co-develop four biosimilars for the Chinese market.

KBI Biopharma, a biopharmaceutical contract development and manufacturing organization, will manufacture an antibody and a fusion protein developed by Heat Biologics’ subsidiary.

FDA rejected the company’s biologics license application for its anti-rheumatoid arthritis biologic and has requested additional clinical safety data.

This latest FDA approval expands the blockbuster anti-cancer biologic’s indications in oncology.
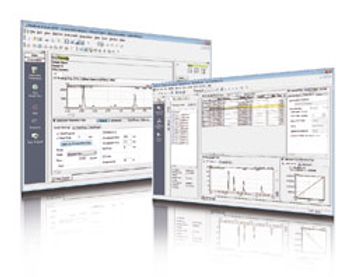
New algorithm and functions in Shimadzu’s LabSolutions software enable compliance with data integrity regulations.
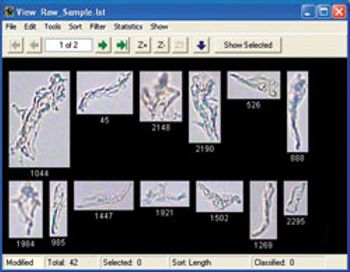
The Nano-flow imaging particle analyzer from Fluid Imaging Technologies automatically image nanoparticles for identification.

Glythera gains exclusive access to Cancer Research UK’s novel CDK11 inhibitor program for the development of ADCs conjugated using its highly stable conjugation platform, PermaLink.

Biopharmaceutical companies developing new competitive biotech therapies have pressed hard for clarity on the testing and data required by FDA to gain market approval of biosimilars that can be filled by a pharmacist without prescriber preapproval.

Ajinomoto Althea opens manufacturing suites in new high potency and antibody drug conjugate commercial facility.

The collaboration with Immunocore, a T cell receptor company, aims to discover and develop immunotherapy molecules to treat infectious diseases.

The approval marks the first biosimilar approved in the United States for treating cancers.

European Medicines Agency’s Committee for Medicinal Products for Human Use grants a positive opinion for the approval of the biosimilar for treating breast cancer.

The new building is protected by special construction against vibration to ensure accurate calibration of laboratory balances.