
Catalent expands the scope of the OptiForm Solution Suite to bridge gap from late-stage discovery to Phase I trials.

Catalent expands the scope of the OptiForm Solution Suite to bridge gap from late-stage discovery to Phase I trials.

Pfizer’s new format of ibuprofen (AdvilR Liqui-GelsR Minis) uses Catalent’s softgel technology to deliver the formulation in a more concentrated fill, resulting in smaller capsules.

Recro Gainsville expands its tableting capacity with the addition of a tablet press and film coater.

Samsung BioLogics signs $55.5 million agreement to manufacture tildrakizumab for Sun Pharma.

As pharma models changed during the past 40 years, contract manufacturing capacity and services evolved to meet demand.

The more pharma science and technology change, the more business and policy concerns stay the same.

Cambrex announces expansions of its North Carolina API pilot plant and Kalskoga, Sweden large-scale manufacturing facility.
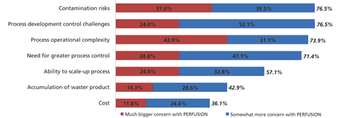
Although widespread adoption of continuous bioprocessing has been slow, some processes have been an exception.

Catalent has signed an agreement with Therachon to support preclinical and clinical development of TA-46, a novel protein being developed to treat achondroplasia.

The company invested approximately $26 million to update its sterile pharmaceutical and development service suites.

CMOs may be gaining as strategic partners to large bio/pharma companies, but they have a much harder path to navigate.

Caladrius is selling the remaining percentage of the subsidiary in order to focus on cell therapy development.

Cold-chain requirements and the tight logistical windows needed for cell and gene therapies demand a focus on early communication and risk mitigation.

Vetter expanded visual inspection facilities and controlled-temperature storage at its Ravensburg Vetter West facility in Germany.

Catalent announces a development agreement with JOT to evaluate softgel options for a resveratrol drug candidate.

Pharma Tech introduced a new high-speed bottling line to its facility in Union, MO.

AGC adds second biopharma contract manufacturer with acquisition of CMC Biologics.

The company has manufactured double-digit number of batches on the new filling line, for use in early-stage clinical trials.

Idifarma has acquired a Bosch GKF-702 capsule filling machine that can manufacture 3000 to 42,000 capsules per hour.

Catalent adds two softgel facilities and packaging capabilities with acquisition of Canada-based Accucaps.

Patheon adds API manufacturing capacity with acquisition of Roche’s Florence, SC facility.

The new facility will focus on formulation development, drug product analytical development, and quality control.

CMO executives are focusing on M&A activity, new business models, and fundraising limits.

Recipharm is investing more than EUR1.2 million to enhance its small-scale GMP API development and manufacturing capabilities in Paderno Dugnano, Italy.

Catalent invests $34 million to add a 2 x 2000-L single-use bioreactor system and laboratory space in Madison, WI.