
PharmSource forecasts slow growth in a new report on the outlook for the contract manufacturing industry.

PharmSource forecasts slow growth in a new report on the outlook for the contract manufacturing industry.
Suppliers indicate prices for single-use equipment are likely to increase.

Recipharm will be responsible for the supply of the remaining clinical-trial material and ongoing future commercial supply of RHB-105, the lead drug candidate developed by RedHill for the treatment of Helicobacter pylori bacterial infection.

Cytovance Biologics anticipates continued expansion plans following acquisition by Hepalink USA

Companies in the US will now have the opportunity to tap into Hermes Pharma’s technical expertise in effervescent and chewable tablets, instant drinks, lozenges, and orally disintegrating granules (ODGs).

CMC Biologics will manufacture monoclonal antibodies (mAbs) and provide process development services for the PATH Malaria Vaccine Initiative.

Catalent selects advisory board members to complement Redwood Bioscience Scientific Advisory Board.

What if the expanding pipeline isn’t enough to fuel CMO growth?

Ignoring a contract partner’s ability to handle highly potent APIs (HPAPIs) safely may have serious consequences. Drug owners and contract service providers alike must understand the complexities and liabilities involved in working with HPAPIs.
Within the past few years, key players have left the sterile manufacturing business. Can new technology and investment revitalize this critical market?
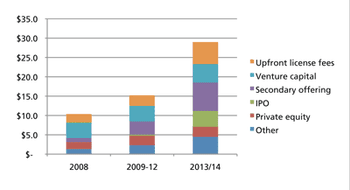
While all market signs are pointing up, memories of past setbacks may discourage from expanding capacity.

Gil Roth, Founder and President of the Pharma & Biopharma Outsourcing Association speaks with Pharmaceutical Technology.

Jim Miller, President of PharmSource, spoke with Pharmaceutical Technology about working with CMOs and CDMOs.

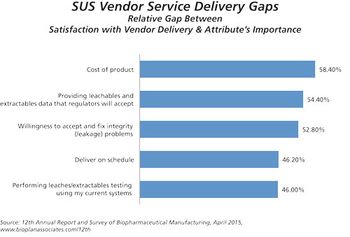
The 12th Annual Report and Survey of Biomanufacturing is now available.

Vetter completes on-site expansion activities of visual inspection and in-process control at Chicago facility.

Grand River Aseptic Manufacturing named one of Michigan 50 Companies to watch.

IDT Biologika receives 2015 Facility of the Year Award or facility integration from ISPE.

The past six months has seen some major changes to the sterile manufacturing landscape in Europe. There have been a number of exits and acquisitions that have no doubt grabbed headlines, but has anything really changed?

A presentation by Jim Miller will offer a detailed review of the contract services landscape.
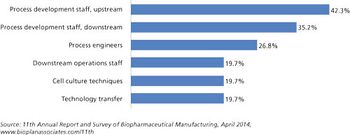
Is there enough talent to go around?

Market forces may limit the success of CMOs.

Nelson Patterson has been elected to Pharma & Biopharma Outsourcing Association board. The Pharma & Biopharma Outsourcing Association (PBOA) has announced that Nelson Patterson, vice-president, sales and marketing at Baxter BioPharma Solutions, has been elected to its Board of Trustees, effective immediately. Patterson was elected after Baxter BioPharma Solutions joined the PBOA as a Sustaining Member.
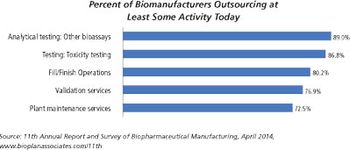
There are significant differences between small molecules and biologics fill/finish capacity.
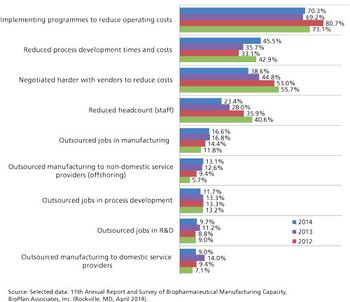
Outsourcing is taking on a greater role in the biopharmaceutical manufacturing industry.

The trend of exits from the CMO industry looks to be gaining momentum.