
The project will foster the translation of nanomedicine applications for the treatment of cardiovascular diseases.

The project will foster the translation of nanomedicine applications for the treatment of cardiovascular diseases.

The agency issued its recommendation for the influenza virus strains European vaccine manufacturers should include for 2017.

In a new study, researchers from Boston Children’s Hospital study responses to pneumococcal vaccine in infant monkeys.

Researchers continue efforts to overcome challenges of effective oral delivery of biologic drugs.

GW Pharmaceuticals plans to submit a regulatory filing to FDA and EMA following two positive Phase III trials of Epidiolex in patients with Lennox-Gastaut Syndrome.
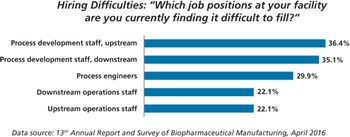
New study shows China biopharma companies face staffing shortages.

The transfer of fluids is governed by different equipment requirements across the medical, biopharma, and cell therapy manufacturing industries.

The process control and automation requirements of single-use systems differ from those of stainless-steel equipment.
Design of experiment plays a crucial role in the optimization process of formulation development.

Anil Kane, executive director, Global Head of Formulation Sciences, Pharmaceutical Development Services at Patheon discusses key parameters in the development and manufacturing of oral solid-dosage forms.
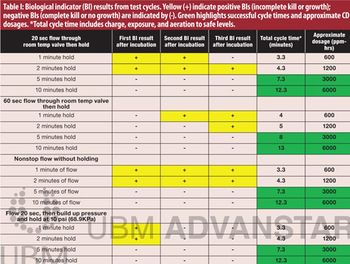
A chlorine dioxide sterilization cycle was developed for a novel split-valve aseptic powder transfer device.

A venture between GEA and Siemens aims to familiarize more pharmaceutical companies with more modern control and continuous processing.

This novel technology was developed in response to challenges involved in conventional manufacturing of multilayer tablets, including in-line control of the tablet weight, the tendency to delamination, direct contact between the two tablet layers, and cross contamination.

This article summarizes the evolution of the viscosity standards and their corresponding applications in the USP−NF compendia.

A case study reviews the reformulation and scale up of high drug load prototype using wet granulation process for a model formulation.

The facility in Britley will now manufacture the company’s automated systems, enabling the company to better serve European markets and shorten the supply chain.

The agency recommended six drugs for approval in March 2017 including treatments for neuroblastoma, heart failure, and more.

In a FDAVoice blog post, CBER Director Peter Marks discusses the new designation for cell therapies that treat life-threatening diseases.

Fisher BioServices will expand its CryoHub solution by co-locating it with the Cell and Gene Therapy Catapult manufacturing center for seamless supply chain management and to accelerate cell and gene therapy production.

Manufacturing will be carried out at the Pfizer Newbridge, Ireland, facility, which is now part of Pfizer CentreOne’s contract manufacturing network.

The ready-to-fill packaging solutions for vials are based on Ompi EZ-fill packaging design.

Parenteral packaging will be well-represented at INTERPHEX, especially technologies associated with fill/finish of ready-to-use vials, cartridges, and syringes.
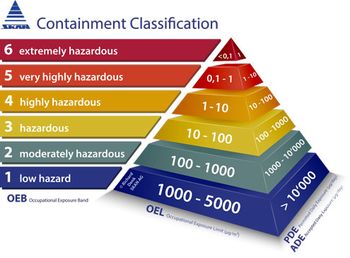
The new ISPE Containment Manual is a summary of the process involved in the manufacture of highly active or highly hazardous pharmaceutical substances.

Effective solutions for overcoming the high molecular weight, hydrophilicity, and instability of large biomolecules have yet to be identified.

Pharmaceutical Technology spoke with Frank Generotzky, plant manager for Baxter BioPharma Solutions’ Halle, Germany facility, about operational excellence at the site.