
The biosimilar pathway permits licensure based on less than full clinical data.

The biosimilar pathway permits licensure based on less than full clinical data.

Oxford Genetics has secured a GBP1 million capital from investment group Mercia Technologies to support its growth strategy in delivering new services for cancer-fighting gene-therapy technologies.

The collaboration follows the signing of a cooperative research agreement between Sanofi Pasteur, Fiocruz, and WRAIR.

FDA’s new Office of Tissues and Advanced Therapies will oversee a growing range of cellular and genetic products.

Catalent invests $34 million to add a 2 x 2000-L single-use bioreactor system and laboratory space in Madison, WI.

Horizon announces new bioproduction outlicense deal with an unnamed partner for a minimum value of £500,000.

HHS entered into a contract with BioProtection Systems Corp. for commercial manufacturing tests of an Ebola vaccine.

WuXi AppTec’s new biomanufacturing facility is its third facility in the Philadelphia, PA Navy Yard.

Multiple inspection techniques facilitate the identification of particulate matter in vials as part of root-cause investigations and safety evaluations.

The Intelligent Control Inhaler is an intuitive, fully-integrated device delivering accurate doses of medication to patients, while providing on-screen instructions for use and feedback to the patient and healthcare provider via an app.

Novasep’s BioSC Lab chromatography is a flexible equipment that enables operations from one column up to six columns in batch, parallel batch, or continuous processing with all media and membrane types.

The winning entry was Merck’s Emprove program, which facilitates risk assessment and supplier qualification by providing instant, online access to regulatory and technical information on hundreds of products used in pharmaceutical and biopharmaceutical manufacturing.

Xellia added laboratory space and personnel in Zagreb, Croatia to work on anti-infective products that combat the antimicrobial resistance problem.

The Koolit Advanced PCM Gel from Cold Chain Technologies provides cold-temperature protection for drugs and vaccines during transport.

A PESU membrane is now available for Sartorius Stedim Biotech Sartocon benchtop and production-scale filtration assemblies.

The hospital received a five-year $5 million grant from CDC to survey for communicable diseases in children and evaluate vaccine effectiveness.

This article will equip the excipient vendor with an understanding of QbD from the perspective of the topical pharmaceutical product manufacturer.
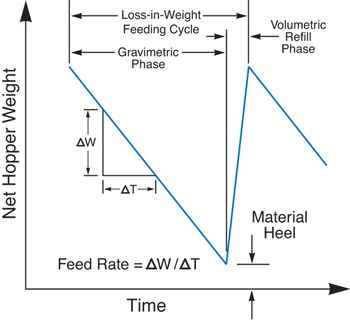
Designing loss-in-weight feeders for accurate and consistent refill is crucial to a continuous solid-dosage process.

Primary packaging and manufacturing technologies minimize product/package interaction, protect quality, support safe travel through the supply chain, and enhance performance at point of use.

Excipients play a crucial role in the manufacturing of solid-dosage forms and the performance of the finished drug product.

Understanding the components of a reference marketed pMDI is needed to develop a generic pMDI.

Ravi Limaye gives an overview of the biosimilar industry and projects for 2020.

Alan Sheppard, Principal, Global Generics at IMS Health and CPhI expert panel member, discusses generic medicine opportunities in the 2016 CPhI Annual Industry Report.

Three-dimensional (3D) printing, which is a type of additive manufacturing (AM), enables fabrication of specialty drugs and medical devices, said Emil Ciurczak, Doramaxx Consulting and CPhI expert panel member, in the 2016 CPhI Annual Industry Report.

Containing costs and improving compliance will be crucial to ensuring the growth of generic pharmaceuticals in the future, says Dilip Shah, CEO of Vision Consulting Group, in CPhI’s 2016 Annual Report, which will be formally issued at CPhI 2016 in Barcelona.