
Language surrounding regenerative medicine and the REGROW Act appeared back into the 21st Century Cures Act right before it passed. What will this mean for the controversial testing and marketing of stem-cell therapies?

Language surrounding regenerative medicine and the REGROW Act appeared back into the 21st Century Cures Act right before it passed. What will this mean for the controversial testing and marketing of stem-cell therapies?

The companies will combine expertise on T-cell therapies with two or more binding domains to create novel oncology medications.

The partnership will focus on providing practical information to clients on the development of biologics and vaccines.

GEA’s ConsiGma continuous tableting line combined with Siemens’ automation and Sipat data management systems enables continuous manufacturing.

Catalent announces a development agreement with JOT to evaluate softgel options for a resveratrol drug candidate.

Moisture uptake during the end-to-end manufacturing process and supply chain can affect product quality. Simulation tools based on mechanistic models help define storage and handling requirements for oral solid-dosage drugs.

Advances in materials and equipment for pharmaceutical blister packaging protect quality and enhance shelf life.

FDA plans to advance initiatives for ensuring reliable production of drugs and biologics in 2017.

A pilot project, beginning in 2017, will support the development of biosimilars.

Univercells received a grant from the Bill & Melinda Gates Foundation for the development of a vaccine manufacturing platform.

Oxford Genetics received £1.61 million from Innovate UK to explore computational and synthetic biology approaches for optimized mammalian bioproduction.

GlaxoSmithKline opened a new vaccines R&D center in Rockville, MD creating up to 200 new jobs.

Principles of dissolution testing, including method development and testing apparatus, are reviewed.

FDA and BARDA awarded a contract to Continuus Pharmaceuticals to develop an end-to-end continuous manufacturing process for solid-dosage drugs.
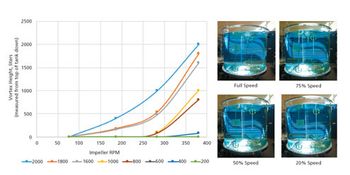
Quantitative and qualitative tools allow better understanding of mixing in a single-use bioprocessing system.
Hot-melt coating was used to develop taste-masked orally disintegrating granules of acetaminophen and caffeine.

Takeda will invest more than 100 million Euros to build a new manufacturing plant for its dengue vaccine candidate in Singen, Germany.

Catalent adds two softgel facilities and packaging capabilities with acquisition of Canada-based Accucaps.

Oxford Genetics has been awarded a grant to develop packaging cell lines for virus bioproduction and will work in collaboration with the University of Oxford to generate cell lines for the scalable manufacture of retrovirus and lentivirus vectors.

Avecia is adding drug substance capacity at its Milford, MA manufacturing site.

Research by agency scientists may help speed development of Zika virus vaccines.

Idifarma has announced a growth of more than 50% over the past two years. Revenue is expected to hit approximately EUR5.3 million in 2016, growing from EUR3.5 million in 2014.

German biotech ARTES and Iranian biopharma manufacturer BioSun will develop an HPV vaccine.

This article will clarify reasonable expectations for the responsibilities of topical product formulation developers and for excipient suppliers regarding the information and samples for experiments needed for QbD.

Reliable, high-quality products require innovative analytics and production.