
Zika vaccine development is hindered by technical challenges and funding shortfalls.

Zika vaccine development is hindered by technical challenges and funding shortfalls.

Topical semi-solids, like creams, lotions and ointments are comprised of a complex mixture of microstructures or colloidal phases, frequently referred to as the Q3. These microstructures may include micelles, lamellar phases, polymer matrices, liquid crystalline and crystalline states of wax excipients, as well as the solid states of active pharmaceutical ingredients. Different topical medicated semi-solid formulations, spanning a range of treatment indications, reveal a variety of different microstructures.
The Claristep filtration system from Sartorius improves preparation of samples for analytical testing.

Catalent Pharma Solutions teamed with Zumutor Biologics to develop antibodies with enhanced ADCC activity.

Jacobs Engineering Group was awarded a contract to provide engineering services and procurement for Alnylam Pharmaceuticals’ new manufacturing facility in Norton, Massachusetts.
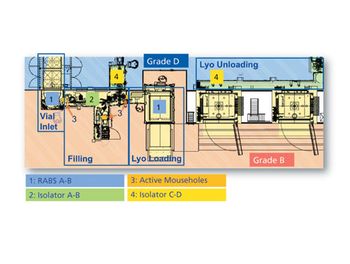
The design of Baxter BioPharma Solutions’ aseptic filling lines provides a case study in customizing containment systems for multi-product lines

Three-dimensional printing allows unique benefits to be built in to solid-dosage forms.

A technology management process identifies and evaluates new technologies in biopharmaceutical manufacturing to aid business decisions.

A Q&A with FDA to gain insight on FDA's views of three-dimensional printing and its regulation in drug manufacturing.

PharmTech’s 2016 survey shows general satisfaction with existing solid-dose and parenteral manufacturing equipment, and slow adoption of continuous manufacturing processes.


An MIT-developed system uses microbes for manufacturing small amounts of vaccines and other therapies.

While isolators may offer advantages in high-speed commercial manufacturing lines, RABS continues to be a flexible alternative solution for small-scale production of drugs for clinical use.

Amgen discussed the drug-delivery approaches to two of its biologics in a recent second-quarter earnings call.

NIST’s monoclonal antibody reference material can be used as a standard for biopharmaceutical analytical quality control.

FDA and industry seek speedy Congressional approval of new user fee plan.

The Phase I trial will test Bavarian Nordic’s vaccine, which is manufactured using a vaccine vector based on smallpox.The recent resurgence of yellow fever incidences over the past six months has prompted health officials to ramp up the fight against the virus. Like the Zika virus, yellow fever is transmitted primarily through the bite of infected female Aedes aegypti mosquitoes.

The affinity purification company will set up shop at the former Merck & Co./GlycoFi facility in the Dartmouth Regional Technology Center in New Hampshire.

The project involves installation of a small-scale pressured agitated nutsche filter dryer in glass, integrated in a high-containment isolator to achieve an occupational exposure limit of less than 1 microgram per meter cube, 8-hour time weighted average.

The vaccine candidate has also won a Priority Medicines (PRIME) status from EMA.

Gamma-stable fluoropolymers are an alternative material for single-use bags and assemblies in biopharmaceutical manufacturing.

The collaboration will focus on the investigational candidate JTX-2011 and up to four other early-stage programs in immune-oncology.

The antisense drug will be the first in the companies’ joint development deal for medications to treat autoimmune disorders of the gastrointestinal tract.

FDA issued a warning letter to the Worthing, UK facility for cross contamination and microbial contamination cGMP violations.

The agency says the increasing requests for orphan drug designation has resulted in a change in FDA’s review goals.