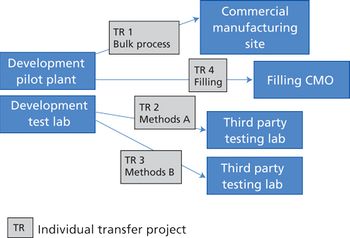
Integrating quality and compliance with technology transfer and careful project management are key in starting up a facility and launching a biologic drug.

Integrating quality and compliance with technology transfer and careful project management are key in starting up a facility and launching a biologic drug.

Early planning for the integration of clean-in-place systems for equipment cleaning is key.

Aseptic spray drying provides an alternative to lyophilization as an enabling stabilization technology for parenteral biologic formulations.

A study by MilliporeSigma and the Economist Intelligence Unit reviews growth drivers and approaches to mitigate risk.

The drug received breakthrough therapy and orphan drug designation as a monotherapy for the treatment of chronic graft-versus-host-disease.

Antibodies to dengue may either confer protective immunity to the Zika virus or enhance disease severity in secondary, related infections such as Zika.

MilliporeSigma and the International Vaccine Institute in Seoul, South Korea aim to develop more robust, scalable vaccine manufacturing processes.

The monoclonal antibody for the treatment of two forms of multiple sclerosis has a target action date of December 28, 2016.

The agency recommends Zalmoxis, a new cell-based therapy to support stem cell transplantation in patients with high-risk blood cancer.

Studying broadly neutralizing antibodies in infants may lead to new pathways in HIV vaccine development.

A research team associated with Dr. Carl June announces it has discovered a way to engineer a patient’s own immune cells to recognize cancer-specific glycoantigens on tumor cells.

A new indication for Emergent BioSolutions’ BioThrax will give the drug market exclusivity through November 2022.

Pfizer broke ground at its Andover, Massachusetts campus on a clinical manufacturing facility for complex biologics and vaccines.

The agency provides quality, development, manufacturing, and labeling recommendations.

Hovione expands drug substance and HPAPI capacity in East Windsor, New Jersey.

A study published in BMJ indicates that rheumatic patients with anti-infliximab antibodies may have a similar cross reaction to infliximab biosimilars.

FDA approved Vaxchora intended for travelers who are at risk for the disease.

Alvotech prepares for commercial biosimilar production in new facility with single-use bioreactors in Reykjavik, Iceland.

The inactivated vaccine, manufactured with a GE FlexFactory system, could be associated with fewer adverse reactions than the live vaccine option.

Pharmaceutical Technology sat down with Charles N. Kettler, PhD, director of Natoli Scientific, to discuss the Natoli Institute for Industrial Pharmacy at Long Island University.

The agency reiterated its earlier decision to require suffixes in biosimilar naming, but was unclear on suffix meaning.
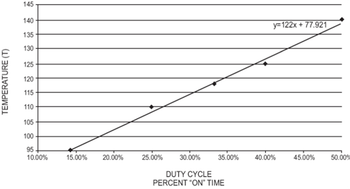
Preheating pinch valves prevents drift in the volume of liquid dispensed.

Industry experts discuss the benefits and challenges of using single-use systems in pharmaceutical manufacturing.

With technology advances, continuous manufacturing shows steady progress to more widespread adoption.

Experts discuss the key considerations in the development of an autoinjector.