
At DCAT's annual meeting, Bill Downey, president of the market research firm High Tech Business Decisions, summarized results from its latest survey on biopharmaceutical outsourcing.

At DCAT's annual meeting, Bill Downey, president of the market research firm High Tech Business Decisions, summarized results from its latest survey on biopharmaceutical outsourcing.

Industry is joining with academia and global health leaders to examine new approaches to biotech manufacturing.

Novo Nordisk broke ground on a facility in Clayton, NC, to manufacture APIs for GLP-1 and insulin medicines.

A team of Bristol-Myers Squibb scientists will work in a new laboratory at the National Institute of Bioprocessing Research and Training facility in Dublin, Ireland.

Collaboration will provide for unified development and manufacture of antibody drug conjugates.

The Human Vaccines Project brings together academic research centers, industrial partners, nonprofit organizations, and governments to address the primary scientific barriers to developing new vaccines and immunotherapies.

During a clinical trial of 48 participants, 100% of those that received the TV003 vaccine were protected from infection.

The author discusses key areas of focus and presents best practices from the International Pharmaceutical Excipients Council (IPEC).

Wear-resistant materials and coatings protect tablet punches and dies from abrasive formulations.

The syriQ Rigid Cap (SRC) and SCHOTT TopPac Rigid Cap (TRC) closure systems from Schott use an intuitive twist-off mechanism for ease of use and container closure integrity.

Hovione will operate a commercial-scale continuous manufacturing facility in New Jersey as part of an agreement with Vertex Pharmaceuticals.
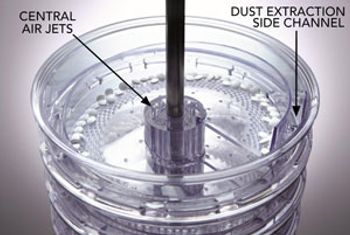
Dedusting equipment and techniques address problems associated with tablet manufacturing dust accumulation.
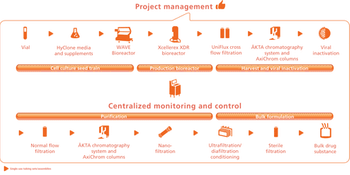
Asking the right questions is crucial to establishing a biopharmaceutical facility design.

The pharma company revealed in a fourth quarter call that it will improve its cell-culture capabilities by focusing on the use of naïve, highly proliferative cells to manufacture its CAR-T drug candidate.

The author lists five key areas to consider when selecting a CDMO to develop highly potent formulations.

Tablet presses require regular inspection and maintenance to prevent premature wear and tableting problems.

Manufacturing of antibody drug conjugates requires high-containment solutions, such as high-performance aseptic isolators.

The course, intended for healthcare professionals, provides an overview of biosimilar products and FDA’s biosimilar product development programs.

Vaccine R&D has grown exponentially in recent years, spurred by ethical and medical needs to combat lethal infectious outbreaks and increased funding from public and private agencies and organizations.

There are no clinically meaningful differences between Celltrion’s CT-P13 and Remicade, according to an FDA briefing released ahead of the formal panel meeting.

Although switching has occurred in European markets for some biosimilars, most biosimilar manufacturers will focus on securing new users, according to Merck.
Expanded systems integration, serialization, and supply chain connectivity will enhance productivity and support counterfeit prevention.
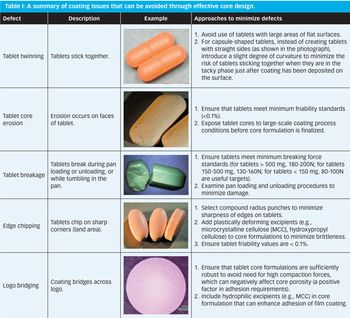
Common defects in tablet film coating can be minimized by effective design of the tablet core and the coating process.

Scale-down modeling is instrumental in supporting the development of downstream biopharma manufacturing processes.

The Biosimilars Forum launched Partnership for Biosimilars Education and Access, an education initiative raising awareness of biosimilars in the US.