
FDA approved 45 novel new drugs in 2015, the highest number of approvals since 1996 and second-highest ever.

FDA approved 45 novel new drugs in 2015, the highest number of approvals since 1996 and second-highest ever.

Ionis Pharmaceuticals receives orphan drug designation for HTTRx for the treatment of Huntington’s disease.
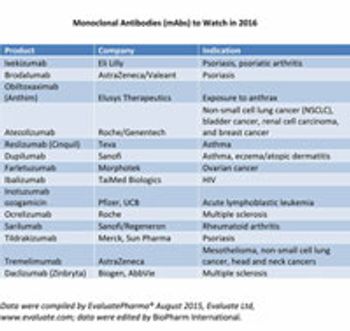
BioPharm highlights the monoclonal antibodies that may gain United States regulatory approval in 2016.
Parenteral packaging of the future will include more automated lines, ready-to-fill packaging formats, and supply-chain transparency.

Infrastructure and payer decisions will determine drug choices in emerging and developed regions.

Amid corporate restructurings, regulatory initiatives, and aging R&D assets, will drug development accelerate or stall in 2016?

FDA approved 45 novel new drugs in 2015, the highest number of approvals since 1996 and second-highest ever.

Regulatory, corporate restructuring, and manufacturing issues will challenge bio/pharma to meet the needs and expectations of patients around the world.

Liquid formulations in hard-shell capsules or softgels are becoming a popular option for HPAPIs because of advantages such as improved safety and lower risk of potential exposure and product cross contamination.

The DF30Plus, a new version of Aptar Pharma’s aerosol metering valve for pressurized metered dose inhalers (pMDIs), incorporates an elastomeric cyclic-olefin-copolymer (COC) neck gasket.

Synthetic biology promises to drive tomorrow’s therapies, while continuous processing is already being used in some new drugs.

Boehringer Ingelheim announced it will establish a new biopharmaceutical production facility in Vienna.

Synpromics announced collaborations with Avalanche Biotechnologies and Applied Genetic Technologies Corporation to use synthetic promoters to develop gene therapies, including adeno-associated virus technology for treating eye diseases.

Collaboration between users and suppliers to increase understanding, clearly define user requirements, and mitigate risk is facilitating acceptance by industry and regulators.

Millipore Express PHF hydrophilic, sterilizing-grade filters from EMD Millipore offer faster flow rates to speed the process or reduce the process footprint.

PSL has installed several advanced contained filtration and drying facilities as part of an expansion of a pharmaceutical manufacturing plant in Singapore.

Sandoz announces that the European Medicines Agency has accepted a MAA for a biosimilar of etanercept.

Seqirus, CSL Limited’s influenza vaccine business, announced the opening of their corporate headquarters in the United Kingdom.

Microorganism lethality requirements for process validation must always be balanced with the need to protect product integrity and patient safety.

Analytical methods and functional assays are used to compare molecules and relate characteristics to quality attributes.

The advantages of fixed-dose combinations are well recognized but their formulation and manufacture can be a challenge, Stefania Barzanti from IMA Active explains why.

Finalizing GMP requirements and quality standards for the development, manufacture, and clinical testing of ATMPs in the EU is proving to be a complex task.

The CEO of a US-based biosimilar manufacturer explains the legal and intellectual property issues of bringing a biosimilar to market in the United States.
Quality by design, in-vitro release testing, and modern analytical methods are improving understanding and control of these complex formulations.

The results of an industry workgroup’s examination of EMA’s guide on shared facilities are presented.