
A US government report on advanced manufacturing promotes continuous manufacturing of pharmaceuticals, which has had recent commercial success but faces challenges for widespread adoption.

A US government report on advanced manufacturing promotes continuous manufacturing of pharmaceuticals, which has had recent commercial success but faces challenges for widespread adoption.

The company’s method reduces the time required to crystallize antibodies from weeks to one day.

Combining powder micro-dosing services with the Xcelodose technology, Capsugel offers capabilities in both North America and Europe.

Collaboration and single-use technologies aided the rapid scale-up of Ebola vaccine manufacturing

The GPhA and its Biosimilars Council expressed concern about the new proposed value-based reimbursement rules for Part B medications.

Phase I clinical trials of PfSPZ revealed it may protect healthy adults, who have not been exposed to Malaria before, for more than on year.
The production of antibody-drug conjugates requires biopharmaceutical and chemical manufacturing, and conjugation capabilities.

MilliporeSigma expands its Carlsbad, California-based GMP capacity for viral and gene-therapy products by nearly 90%.
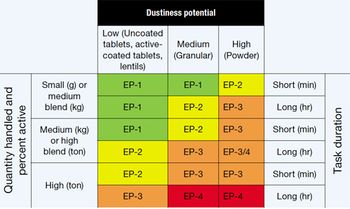
Safe handling of HPAPIs requires determining exposure potential and selecting appro-priate containment strategies.

Experts discuss some of the emerging trends in bioprocessing in 2016, including 4D bioprinting, 2D-NMR, and the CAR-T design space.

Bioprocess operations-from cell line selection to final filtration-can influence the consistency and purity of biologic drug substances.

Policies for patient access to life-saving therapies must keep pace with biomedical innovation.

Armin Gerhardt, associate professor of Pharmaceutical Science, Concordia University Wisconsin School of Pharmacy, discusses the effects of moisture on product quality and how to achieve good control of moisture during pharmaceutical manufacturing operations.

The agency found no differences in risk of pneumonia for different products.

The company announced the launch of its first-in-class Lynx CDR connectors at INTERPHEX 2016.

Highly potent or cytotoxic drugs require special handling

The Subcommittee for Advanced Manufacturing of the National Science and Technology Council highlights biopharmaceutical manufacturing as an emerging priority.

Traditional methods of measuring the effectiveness of vaccines against the flu are called into question by new findings from the NIH.

Linker technology and drug combinations play an important role in the efficacy of ADCs.

The Office of Generic Drugs highlights the agency’s work to advance generic drugs.

FDA approved an update in the manufacturing of Prezista (darunavir) using a continuous manufacturing line at Janssen Supply Chain’s facility in Puerto Rico.

The agency has announced the creation of the Combination Products Policy Council to address issues related to combination products.

Texture analyzers can be used to evaluate wall hardness and elasticity of softgel capsules.

Consider the 3 Ps (product, process, and patient) when choosing a parenteral packaging material.

Even though the organizers of New York’s Tribeca Film Festival decided not to air the anti-vaccine film by discredited British researcher Andrew Wakefield, the so-called documentary is getting a healthy run and further perpetuating the myth of a link between childhood vaccination and autism.