
The European Pharmacopoeia defines the format and content of monographs for biologicals to keep pace with recent approaches and meet the needs of its users.

The European Pharmacopoeia defines the format and content of monographs for biologicals to keep pace with recent approaches and meet the needs of its users.

Mylan announces a recall of eight lots of injectable products due to visible foreign particulate matter.

FDA releases long-awaited guidance documents regarding the assessment of biosimilarity.

Catalent’s Zydis technology will be used to develop thermo-stable and cold-chain independent vaccines.

The agency has recommended granting marketing authorization for Opdivo.

Dicerna Pharmaceuticals announces that FDA granted its primary hyperoxaluria type 1 (PH1) treatment Orphan Drug designation.

Originator product manufacturers will have to update and improve their processing platforms to stay competitive with the biosimilars coming to market.

A presentation by Jim Miller will offer a detailed review of the contract services landscape.

GSK notifies CDC and FDA that it is recalling the remaining doses of its 2014–2015 flu vaccine, Flulaval Quadrivalent Thimerosal-free Pre-Filled Syringes, due to decreased potency.

PTI's next-generation VeriPac Inspection System, the VeriPac 310, uses new vacuum-decay technology to increase leak-detection accuracy for a range of flexible and rigid packages and containers.

The acquisition expands Sartorius Stedim Biotech’s service portfolio.

A core coating module allows Roeltgen's FlexiTab development and small-batch tablet press to manufacture multi-layer and externally lubricated tablets.

The Ph. Eur. contemplates adding specifications for sub-visible particles in eye drops and eye lotions to its monograph.

Recipharm makes a strategic investment in Synthonics and partners in development of novel compounds.

Innovations include multimedia packaging and improved filling systems.

The single-use clarification system eliminates centrigues; harvesting can be performed in one step; and process robustness and predictability are ensured.

Bosch Packaging Technology's RAN 3080 exterior washing machine removes product residue and other contamination from filled and closed glass containers using a new, efficient, high-pressure cleaning process.

Innovations for tablet tooling and presses improve quality and productivity.

GlaxoSmithKline announces global vaccines research and design facility to be based in Rockville, MD, USA.

Industry awaits the final revision of USP General Chapter and the impact it will have on the evaluation of sterile product package integrity.
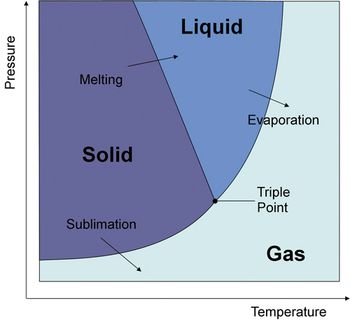
Efficient freeze-drying processes result in time and energy savings, reduced failure rates, and improved batch consistency.

Quality-by-design tools improve efficiency in scale-up of pharmaceutical processes.
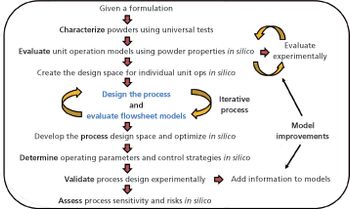
In-silico design facilitates process optimization and evaluation of process control strategies.

NIH announced positive safety results from the vaccine, VSV-ZEBOV, and found that all patients in the study experienced a strong antibody response.
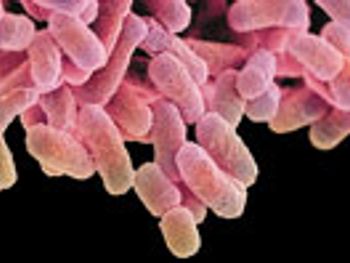
The Human Microbiome Project has increased our understanding of the relationship between humans and microorganisms. The authors offer a new perspective on how this knowledge should be considered in setting standards for pharmaceutical quality control in microbiology.