
The new column features Natrix’s signature macroporous hydrogel.

The new column features Natrix’s signature macroporous hydrogel.
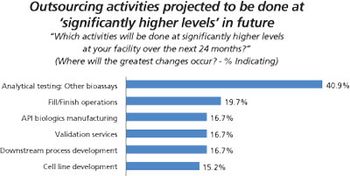
More biopharma companies choose outside service providers for assay testing.

The divestment of Nimenrix and Mencevax was to satisfy regulatory clearances when GSK gained Novartis’ vaccines business in an asset swap in March 2015.

ACG Worldwide discusses current and future uses of capsules in a video interview with Pharmaceutical Technology.

L.B. Bohle discussed tablet coating in a video interview with Pharmaceutical Technology.

The agency clarifies its requirements for allowable excess volume and labeled vial fill size in injectables and biologics.

Reach Separations has announced plans to double its laboratory space at its Nottingham facility as a result of increased demand for its specialist purification services.

Monoclonal antibodies can facilitate the entry of radiopharmaceuticals into cells, David Scheinberg said at BIO 2015.

The agency takes action against websites that illegally sell unapproved medications.

Biomax Informatics AG and Pierre Fabre announced that they have entered into a collaboration to create an integrated antibody sequence and knowledge database.

The agency publishes guidance on the physical attributes of generic tablets and capsules.

Presenters at IVT's Microbiology Week discussed best practices and recent guidance publications for microbial control in sterile and non-sterile pharmaceutical processes.

Experts at Eppendorf discuss common challenges in cell culture and share insights on possible solutions.

Nic Michel, General Manager of Pharma Technology, Inc., spoke with Pharmaceutical Technology about the application of external lubrication in tablet production.

Designed with safety standards in mind, flowtherm Ex incorporates a number of connectable sensors, such as vane wheel, vortex, thermal, Pt100, and other physical sensor with analog output, thereby allowing a range of applications.

The design of the shovel means that less drive power is required compared with a standard shovel, thus resulting in substantial energy savings.

Bosch’s market launches at the show include a new bioreactor for laboratory-scale API development, a water-for-injection unit, and a new generation of pure steam generators with high yields.

Hüttlin GranuLean offers a compact solution for high-quality granulation processes.

Products on display include the newest addition to the Mobius line of single-use bioreactors; Cellvento CHO cell culture media for optimized production of specific cell lines; and new high-volume AFS water purification systems.

Porvair has developed a 2-ml 96 round-well deep-well plate that enables scientists to use the full 2.00 ml well capacity.

GEA’s tableting technologies include a unique system that independently and simultaneously measures and controls both tablet weight and hardness, and a weight control system that provides increased sensitivity at lower forces.
![Image_1_PSL_Dispensary_Isolator[1].jpg](https://cdn.sanity.io/images/0vv8moc6/pharmtech/bf6523e0f089da35fe8d74a4afd23f9b90ee39f2-1000x831.jpg?w=350&fit=crop&auto=format)
PSL has developed a dispensary isolator with a contained drum hoist mechanism for highly potent API production.

This latest model is said to offer higher output and more efficient dedusting process.

GEA will showcase the ConsiGma continuous coater and the PCMM (portable, continuous, miniature, and modular) manufacturing technology platform for continuous production of oral solid dosage forms.

In a Citizen Petition to FDA, AbbVie calls the current biosimilar labeling practices “legally unsound.”