
Engineered containment performance testing is a more robust method for validating containment systems than worker-exposure measurement methods.

Engineered containment performance testing is a more robust method for validating containment systems than worker-exposure measurement methods.

Potential for improved product quality and cost/time savings is reviving interest in perfusion technology.

Currently, pharma has only scratched the surface of the potential value of repurposing drugs, says consultant Hermann Mucke. Contract service partners are playing a more prominent role, but could play an even larger one in these efforts.

Teva Parenteral Medicines initiates voluntary nationwide recall of select lots of Adrucil due to particulate matter.

The Generic Pharmaceutical Association and the Biosimilars Council wrote an open letter to President Barack Obama protesting the patent protections outlined in the Trans-Pacific Partnership.

FDA approved the drug for a more narrow indication in the US than did EMA for Repatha, Praluent’s fiercest competitor.

EMA gives GSK’s malaria candidate vaccine a positive opinion for the prevention of malaria in young children in sub-Saharan Africa.

Investment at Capsugel’s Edinburgh, Scotland facility will expand the company’s liquid- and semi-solid-fill capsule manufacturing capacity.

A Federal circuit appeals court ruled that a biosimilar manufacturer will have to wait 6 months after FDA approval to commercially launch its medication.

GSK accelerates delivery timeline for US quadrivalent flu vaccine.

Improved characterization of twin-screw extrusion enables increasing use in pharmaceutical manufacturing.

The new sizes follow the 2014 launch of the company’s fully disposable purifier.

The European Medicines Agency reviews the safety of human papillomavirus vaccines.

Sepha's VisionScan Max can leak-test full production batches of blister packs.

Teva has launched the generic version of almotriptan malate (Axert), 6.25 mg and 12.5 mg, in the United States.

Vacuum-conveying conditions for powders and other free-flowing solid forms can be simulated in Volkmann's new laboratory.

A study now underway on vaccine manufacturing examines the effects of focusing on consistency during manufacturing, instead of post-production testing.

FDA orders unapproved prescription ear drop products with active ingredients removed from the market.

Biogen plans to build a biologics manufacturing plant in northwest Switzerland using next-generation technologies to create efficiency and sustainability.

Electronic pharmaceutical tablet counters meet demands for accuracy, flexibility, speed, compact size, easy cleanability, and quick changeover.

Anton Paar’s MCP polarimeters are now designed with a new LED light source and optional integrated air pump to clean and dry sample cells.
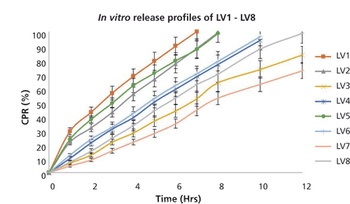
Delivery systems that allow drugs to be administered as liquids, but to form gel within the eye, promise to improve efficacy and patient compliance.

The addition of a new manufacturing line at Lonza’s Portsmouth, NH site enables Alexion to add dedicated product supply for 10 years.

The new facility expands the company’s commercial manufacturing capability at its Bend, Ore. site.