The European Medicines Agency has added granularity to its biosimilars approval pathway by releasing a guideline on biosimilar monoclonal antibodies (mAbs).

The European Medicines Agency has added granularity to its biosimilars approval pathway by releasing a guideline on biosimilar monoclonal antibodies (mAbs).

New systems that combine Raman spectroscopy with automated imaging support the efficient gathering of such data, including information concerning size and shape distributions for individual components within a formulation.

AET BioTech and BioXpress Therapeutics have entered into an agreement to codevelop a biosimilar version of Abbott's tumor necrosis factor inhibitor monoclonal antibody adalimumab.

Potency is a required measurement to determine the amount of active ingredient contained in a preclinical dose formulation. Assessing potency ensures that the test system receives the appropriate amount of active ingredient based on predetermined specifications.

Particle-engineering technologies, such as crystal design for crystallization and producting cocrystals, particle-size reduction, and amorphous solid dispersions, help to optimize delivery of a drug.

Fluid-Bag Ltd provides comprehensive 900 and 1000 litre flexible IBC systems for liquid and semi-solid products, including filling and discharging equipment. The GMP compliant container system is designed to guarantee uniform liquid, maximise payload and minimise discharge residue (0.5% residue).

The long-awaited patent cliff that has loomed in the pharmaceutical industry for years has arrived in earnest in 2012, with more than $40 billion in 2011 brand sales facing loss of exclusivity.
This study examines the effect and interaction of variations in hypromellose physicochemical properties.

The promise of the Generic Drug User Fee Amendments of 2012 is to end multiyear reviews of new generic drugs and the ever-growing queue of pending applications.

The European Medicines Agency has updated its guidance on biosimilar medicines, with the aim of helping companies to avoid unnecessary repetition of clinical trials.

The physical form of an API is an important consideration in formulation development. Particle-engineering technologies, such as crystal design for controlling crystallisation and producing cocrystals, particle-size reduction and amorphous solid dispersions, help to optimise delivery of a drug.

Orphan drugs achieved blockbuster status in 2011, generating over $50 billion, and have the potential to generate as much lifetime revenue as drugs used for more common health conditions, according to a report from Thomson Reuters’ IP & Science division.

As a result of the passage of the Generic Drug User Fee Amendments Act, Janet Woodcock, director of the Center for Drug Evaluation and Research, announced her plan to reorganize the Office of Generic Drugs (OGD) into a super office that would include subordinate offices. The new super office would report directly to Director Woodcock, with Greg Geba continuing his role as OGD director.
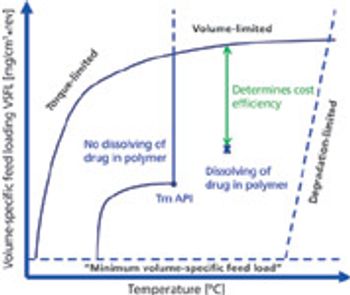
The advantages and disadvantages of hot-melt extrusion in solid dispersion formulations.
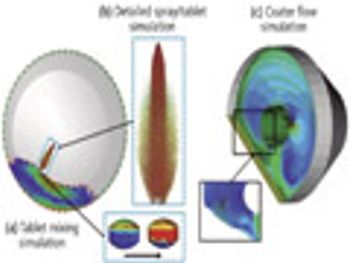
The authors investigate the tablet-coating process using a combination of different simulation techniques.

The authors describe a solid form technology platform used to optimize salt selection, cocrystallization identification and modification, or the development of a free form.

Pfizer has two manufacturing facilities in Germany for high-potency manufacturing, respectively in Freiburg and Illertissen. Pharmaceutical Technology's Executive Editor Patricia Van Arnum visited the facilities and spoke to the company about the design and operation of these facilities.
There is no harmonized guidance on pre-use integrity testing of sterilizing filters, prompting discussion among users as to whether such testing is necessary.

Rob Blanchard and Clive Roberts discuss the issues surrounding tablet sticking.

Pfizer and Mylan have agreed to establish an exclusive long-term collaboration to develop, manufacture, distribute, and market generic drugs in Japan. The products included in the collaboration are expected to be sold under the Pfizer brand with joint labeling.

A look at elastomer changeout times shows how industry knowledge improves operations and cost.
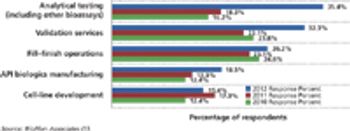
Budgets for biopharmaceutical activities are gaining in select functional areas except outsourcing.

Meticulous system configuration can prevent machines from taking over.
An examination of the current and projected market for biosimilars, development costs for biosimilars compared with small-molecule generic drug, and partnerships in biosimilars.

MedImmune, the global biologics arm of AstraZeneca, announced that it is restructuring its infectious disease and vaccines R&D and operations. This will result in the closure of MedImmune's sites in Mountain View and Santa Clara, California.