
The EMA's Committee for Medicinal Products for Human Use (CHMP) has recommended Sanofi's six-in-one pediatric vaccine for marketing authorization.

The EMA's Committee for Medicinal Products for Human Use (CHMP) has recommended Sanofi's six-in-one pediatric vaccine for marketing authorization.

Merck and Samsung Bioepis have formed an agreement to develop and commercialize multiple prespecified and undisclosed biosimilar candidates.

Protein Sciences announced that the Biomedical Advanced Research and Development Authority (BARDA) will continue to support the company?s influenza vaccines program.
The share of biologic-based drugs in the global pharmaceutical market is on the rise.

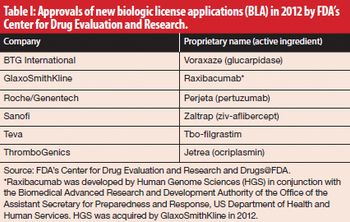
New product reviews for February 2013.
Adopting a seven-step process to maintenance and storage improves tableting quality.

Even in an industry in which all product development is complicated by the intricacies of human biology, orally inhaled products (OIP) stand out as singularly demanding.

GlaxoSmithKline has formed a joint venture with Indian vaccines company Biological E to research and develop a six-in-one combination pediatric vaccine to help protect children in India and other developing countries from certain infectious diseases.

FDA has approved Protein Sciences's FluBlok, a seasonal influenza vaccine made with novel technology. FluBlok uses recombinant DNA and a modified baculovirus (a virus that infects insects) to produce a safe and effective human flu vaccine. FDA approved Flublok for people 18–49 years old.

Adopting a seven-step process resolves tableting problems.

Micronization can be performed with a jet mill or bead mill.

The PATH Malaria Vaccine Initiative and Inovio Pharmaceuticals have announced a follow-on collaboration to advance the development of malaria vaccines and new vaccine delivery technologies.

Janssen Pharmaceuticals, Inc. and GlycoVaxyn AG have entered into a three-year agreement to collaborate on the research and development of a multivalent bacterial vaccine utilizing GlycoVaxyn's bio-conjugation technology.
Is process-centered organization in biopharmaceutical manufacturing a stepping stone or a stumbling block?

PharmTech speaks to Ray O'Connor from the National Institute for Bioprocessing Research and Training (NIBRT) for an overview of aseptic processing.

Vaccine manufacturing was predicted in 2008 to be on the cusp of a golden era, but instead the industry has experienced cost pressures, high-profile liability cases and production setbacks.

Dr. Charles Kettler, director of Natoli Scientific, looks at the challenges that tablet scoring poses to tablet manufacturers.

Tim Freeman of Freeman Technology explains how new analytical technologies have influenced quality criteria and standards for the uniformity of dosage units, and why more accurate systems are leading to greater focus on tablet scoring.

When splitting unscored tablets, the main concerns relate to API dosage control and the potential modification of time-release characteristics. PTE speaks with tableting experts Thierry Menard, Lab Manager, And Bruno Villa, President, both at Medelpharm, about how manufacturers are approaching the challenges.
Tablet splitting is a new area of focus for regulators. The FDA tells PTE more about the challenges in this area.

AAIPharma Expands Parenteral Drug Capacity; Novartis Buys Dendreon Plant; and More.

Room-temperature sterilization using nitrogen dioxide gas provides benefits for sterilizing the external surfaces of single-dose, parenteral drug containers.

Plastic is finding increased use in vials and syringes as concerns about glass breakage and delamination and desire for increased functionality lead pharmaceutical companies to consider alternatives.

GlaxoSmithKline recently developed a novel technology for the formulation of modified-release tablets. The authors describe the route from development to commercialization.

Novartis announced that it has received FDA approval for a seasonal influenza vaccine produced in cell culture, the first seasonal vaccine produced by this method to be approved in the US.