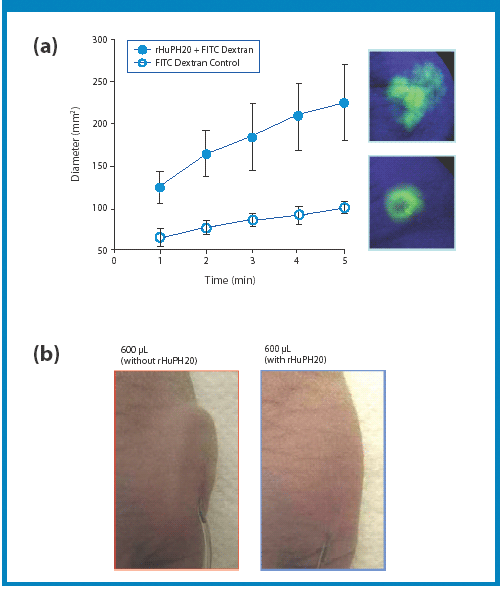
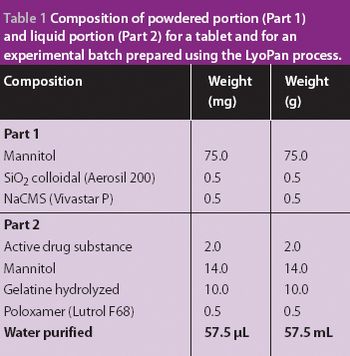
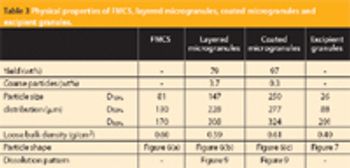
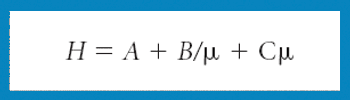A new Raman spectroscopic method to detect magnesium stearate in powder blends and tablets is described. High-volume pharmaceutical manufacturing requires the use of lubricants to facilitate tablet ejection from compressing machines. However, lubricants may also bring a number of undesired problems that have been widely documented in pharmaceutical scientific literature. New analytical methods are needed to understand lubrication and provide process knowledge in support of FDA's process analytical technology initiative. The detection of magnesium stearate in lactose, mannitol, corn starch and other commercially important excipients is reported. The Raman spectroscopic method has a detection limit of about 0.1% (w/w) based on the 2848 cm-1 band that corresponds to the symmetric stretch of the methylene group in magnesium stearate.