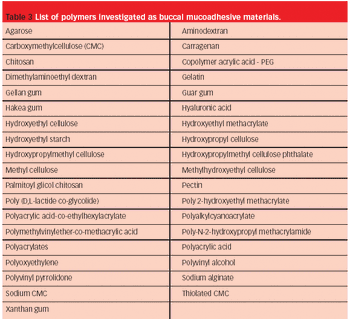
The adequate absorption and transport of drugs in the body is part of optimal therapy. Drug administration perorally is easy, common and traditional, but occasionally alternative routes are required.

The adequate absorption and transport of drugs in the body is part of optimal therapy. Drug administration perorally is easy, common and traditional, but occasionally alternative routes are required.

The assembly of the World Health Organisation (WHO) later this month is expected to feature counterfeit medicines as one of its important discussion points, and there is some hope that the ministers will agree on measures that will strengthen anticounterfeiting legislation and enforcement worldwide.
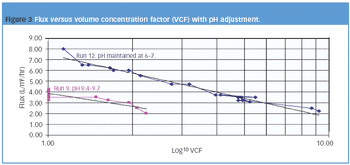
The pharmaceutical industry must address the release of nonbiodegradable APIs into the environment.
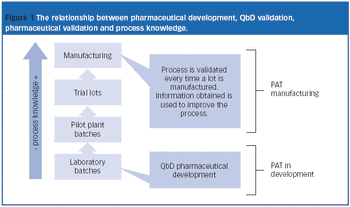
When Pharmaceutical Technology Europe was established 20 years ago, PAT was not a hot topic in the industry. It was started in 2002 by FDA to modernize pharmaceutical manufacturing and increase the efficiency of manufacturing processes.
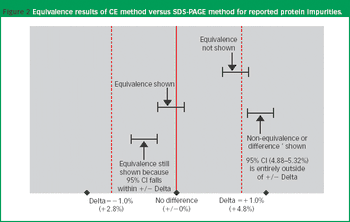
Practical guidance on how to handle validation failures cannot be found in the existing literature because they are not supposed to happen.

Traditional tablet presses do not measure tablets' tensile strength, yet this characteristic strongly influences tablet quality. The author describes a compression technique that accounts for tensile strength and produces tablets with consistent weight and disintegration time.

Congress is taking on the issue of inspection of foreign drug-manufacturing facilities as it began hearings on a discussion draft of the Food and Drug Administration Act of 2008.

Also, Genzyme to build R&D center in Beijing, Noven Pharmaceuticals appoints Peter C. Brandt president and CEO, more...

Problems associated with contamination of heparin products continue after worldwide recalls in March in the United States, Italy, France, and Denmark.

Also, GSK expands in Ireland, executive appointments at ProGenTech, more...

The US Food and Drug Administration issued a final guidance last week regarding investigational new drug applications for human gene therapy.

A manufacturing line can be improved if technology transfer is implemented thoughtfully. Effective technology transfer helps to provide process efficiency and control and maintain product quality.

MannKind suspended discussions with potential partners for the commercialization of its "Technosphere Insulin" product.

Also, Alcon plans to open Singapore facility, Pharmacopeia president and CEO retired, more...

Also, Jubilant Organosys to acquire DRAXIS Health, PPD's Paul Covington to retire, more...

Also, VaxGen and Raven terminate merger agreement, Darren Head appointed CEO of Cytovance, more...

The former formulation-development business of MDS Pharma Services seeks to build its niche in early-phase drug development.

A reference book omits important information and ignores advanced testing procedures.

The economic case for personalized medicine offers a solution to the industry's recent woes.

A changing regulatory environment is on the horizon for excipient suppliers and users.
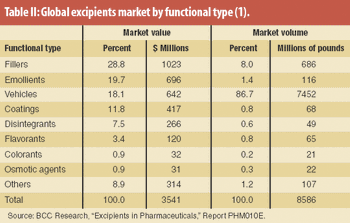
Moderate growth is projected for the global excipients market. Excipient producers target blends and new grades for improving functionality and performance.

The FDA itself issues a cry for help. Is anybody listening?

Excipient producers and industry observers share their perspectives on innovation for excipients.

Antibodies are highly specific molecules that can be tailored to recognize almost any stretch of peptide that nature can conjure: a feature that has been exploited for years now to produce therapeutic antibodies.

What makes a drug ripe for respiratory delivery?